No products in the cart.
Azitrox, 500 mg capsules 2 pcs
€6.00 €5.42
Description
Pharmacotherapeutic group: antibiotic azalid
ATX code: [J01FA10]
Pharmacological properties
Pharmacodynamics
Mechanism of action
Azithromycin binds to the 23S rRNA 50S-subunit ribosomes of susceptible microorganisms. It blocks protein synthesis by inhibiting the transpeptidation/translocation stage and inhibiting assembly of the 50S-subunit of the ribosome.
In vitro, azithromycin is concentrated in phagocytes and fibroblasts. The ratio of intracellular to extracellular concentration >30 after 1 h of incubation. In vivo studies show that concentration in phagocytes can promote spread to inflamed tissues.
The mechanism of resistance
The most common mechanism of resistance to azithromycin is modification of 23S rRNA at positions corresponding to A2058 and A2059 (in the Escherichia coli numbering system). In addition to cross-resistance with other macrolides (erythromycin and clarithromycin), ribosomal modification can determine resistance to other classes of antibiotics (lincosamides and streptogramines B), which bind to overlapping ribosomal sites.
Asithromycin shows activity against most strains of the following bacteria, both in vitro and in clinical infections: Gram-positive bacteria – Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes; Gram-negative bacteria – Haemophilus ducreyi, Haemophilus influenzae, Moraxella catarrhalis, Neisseria gonorrhoeae; other bacteria – Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae.
Asithromycin demonstrates in vitro minimum inhibitory concentrations (MIC) ≤4 µg/mL against most (≥90%) strains of the following bacteria; however, the safety and efficacy of azithromycin in the treatment of clinical infections caused by these bacteria have not been established in adequate and well-controlled studies.
The Gram-positive bacteria are beta-haemolytic Streptococci (Groups C, F, G), Viridans group streptococci; Gram-negative bacteria are Bordetella pertussis; anaerobic bacteria are Peptostreptococcus species, Prevotella bivia; other bacteria are Ureaplasma urealyticum, Legionella pneumophila.
Preclinical toxicology
Carcinogenicity, mutagenicity, effects on fertility. No long-term animal studies have been conducted to evaluate the carcinogenic effects of azithromycin.
Asithromycin showed no mutagenicity in standard laboratory tests: a test on mouse lymphoma cells, micronucleus tests on human lymphocytes and mouse bone marrow.
There is no evidence of impaired fertility associated with azithromycin.
Toxicology in animals
Phospholipidosis (intracellular accumulation of phospholipids) has been observed in some tissues of mice, rats and dogs receiving multiple doses of azithromycin. This has been demonstrated in many organ systems (e.g., eyes, dorsal root ganglia, liver, gallbladder, kidney, spleen, and/or pancreas) in dogs and rats receiving azithromycin at doses that, in terms of body surface area, were approximately equal (in dogs) or approximately 1/6 (in rats) of the recommended adult dose. This effect has been shown to be reversible after discontinuation of azithromycin. Similarly, phospholipidosis was observed in the tissues of newborn rats and dogs receiving daily doses of azithromycin for 10 to 30 days. Based on pharmacokinetic data, phospholipidosis was observed in rats (30 mg/kg dose) with an observed plasma Cmax of 1.3 µg/ml (6 times the observed Cmax of 0.216 µg/ml in pediatric patients at the 10 mg/kg dose). Similar data were obtained in dogs (10 mg/kg dose) with an observed serum Cmax of 1.5 mcg/mL (7 times the observed Cmax at the dose used in the pediatric population). When converted to mg/m2 body surface area, the 30 mg/kg dose for newborn rats (135 mg/m2) and the 10 mg/kg dose for newborn dogs (79 mg/m2) are approximately 0.5 and 0.3, respectively, of the recommended dose in pediatric patients with an average body weight of 25 kg. Phospholipidosis, similar to that seen in adult animals, is reversible after discontinuation of azithromycin treatment. The significance of these results in animals and humans is unknown.
Heart electrophysiology
QTc interval prolongation was studied in a randomized, placebo-controlled, parallel-group study involving 116 healthy volunteers who received chloroquine (1000 mg) alone or in combination with oral azithromycin (500, 1000 and 1500 mg once daily). Combined use with azithromycin increased the QTc interval in a dose- and concentration-dependent manner. Compared with chloroquine alone, the maximum mean (95% upper CI) increases in QTcF were 5 (10), 7 (12), and 9 (14) ms when azithromycin 500, 1000, and 1500 mg were used concomitantly, respectively.
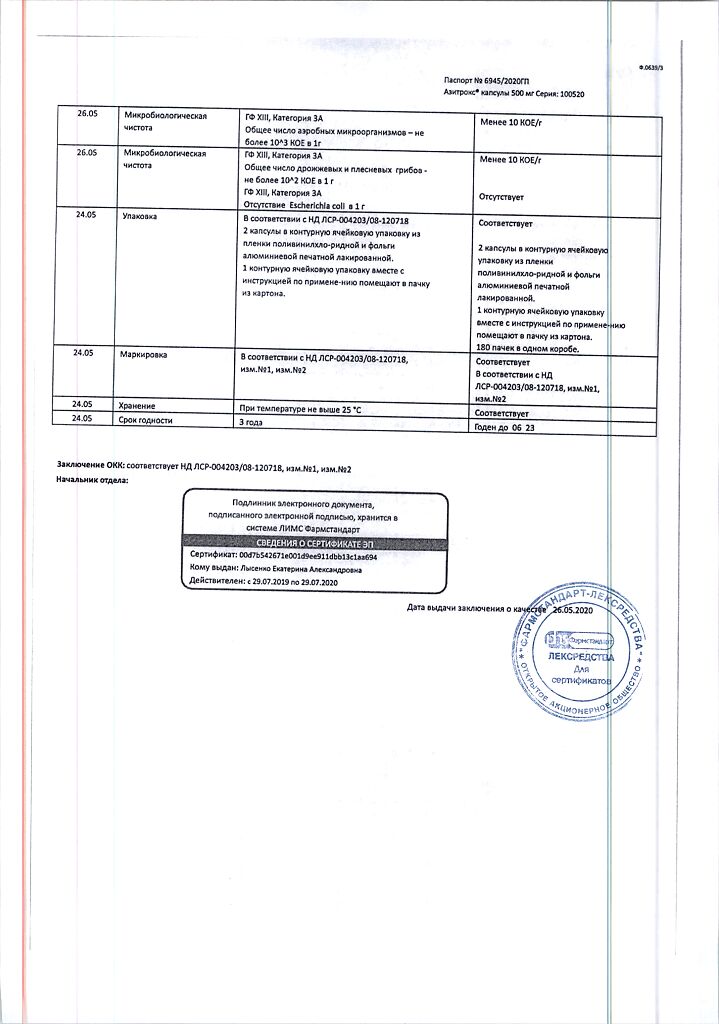
Clinical Studies
PEDIATRIC PATIENTS
The results in pediatric clinical trials were evaluated on days 11-14 because of the increased T1/2 of azithromycin. Data from days 11 to 14 are presented for clinical guidance. An assessment on days 24-32 was considered the primary endpoint of cure.
Acute otitis media
Safety and efficacy of azithromycin at a dose of 30 mg/kg for 5 days
Protocol 1. In a double-blind, controlled clinical trial of acute otitis media conducted in the United States, patients received azithromycin (10 mg/kg on day 1, then 5 mg/kg on days 2-5) or another antibacterial drug. For patients who underwent clinical efficacy assessment (n=553), the clinical success rate (i.e., cure + improvement) on day 11 of the visit was 88% for azithromycin. For patients evaluated at the 30th day visit (n=521), the clinical success rate for patients receiving azithromycin was 73%.
The safety analysis in this study found that the incidence of treatment-related adverse events, primarily gastrointestinal events, in patients receiving azithromycin was 9%. The most frequent side effects were diarrhea/liquid stool (4%), vomiting (2%), and abdominal pain (2%).
Protocol 2. In a noncomparative clinical and microbiological study conducted in the United States, significant levels of beta-lactamase-producing organisms (35%) were found and clinical efficacy was evaluated in 131 patients. The combined clinical success rate (i.e., cure and improvement) at day 11 of the visit was 84% for patients receiving azithromycin. For the 122 patients who were evaluated at the 30th day visit, the clinical success rate for patients receiving azithromycin was 70%.
Microbiologic evaluation was performed at the visit before the start of treatment. No repeat microbiologic evaluation was performed at follow-up visits.
The estimated bacterial/clinical cure results (i.e., clinical success) obtained in the evaluable group at days 11 and 30 were as follows: S. pneumoniae 61/74 (82%) and 40/56 (71%), H. influenzae 43/54 (80%) and 30/47 (64%), M. catarrhalis 28/35 (80%) and 19/26 (73%), S. pyogenes 11/11 (100%) and 7/7, all 177/217 (82%) and 97/137 (73%).
The safety analysis in this study showed that the incidence of treatment-related side effects, primarily from the GI tract, was 9% in all treated patients. The most frequent side effect was diarrhea (4%).
Protocol 3. In another controlled comparative clinical and microbiological study conducted in the United States, azithromycin was used to treat otitis media. There were 2 investigators in this study that participated in the other trial (Protocol 2), and these 2 investigators included 90% of the patients in Protocol 3. For this reason, Protocol 3 was not considered an independent study. Significant numbers of beta-lactamase-producing organisms were found (20%). Ninety-two (92) patients were evaluated for clinical and microbiological success. The combined clinical success rate (i.e., cure and improvement) in patients with the initial pathogen on day 11 of the visit for patients receiving azithromycin was 88% and 82% on day 30.
Microbiologic evaluation was performed at the visit before the start of treatment. No repeat microbiological evaluation was performed at subsequent visits.
The estimated bacterial/clinical cure results (i.e., clinical success) obtained in the evaluated group at days 11 and 30 were as follows: S. pneumoniae 25/29 (86%) and 22/28 (79%), H. influenzae 9/11 (82%) and 8/10 (80%), M. catarrhalis 7/7 and 5/5, S. pyogenes 2/2 and 2/2, all 43/49 (88%) and 37/45 (82%). In the safety analysis in the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients receiving azithromycin was 4%. The most frequent side effect was diarrhea/iodine stools (2%).
The safety and efficacy of azithromycin at a dose of 30 mg/kg for 3 days
Protocol 4. In a double-blind, randomized controlled, comparative clinical trial (n=366) of acute otitis media in pediatric patients aged 6 months to 12 years, patients received azithromycin (10 mg/kg/day for 3 days), a comparison drug, and placebo.
For the 366 patients in whom clinical efficacy was evaluated at day 12 of the visit, the clinical success rate (i.e., cure plus improvement) for azithromycin was 83%. For the 362 patients who were evaluated on visit day 24-28, the clinical success rate for azithromycin was 74%.
In the safety analysis of the aforementioned study, the incidence of treatment-related adverse events, predominantly gastrointestinal, in all patients receiving azithromycin was 10.6%. The most frequent side effects while taking azithromycin were diarrhea/iodine stools (5.9%) and vomiting (2.1%).
The safety and efficacy of a single dose of 30 mg/kg azithromycin
Protocol 5. A double-blind, randomized controlled trial was conducted at 9 clinical sites. Pediatric patients aged 6 months to 12 years were randomized in a 1:1 ratio to treatment with either azithromycin (at a single dose of 30 mg/kg on day 1) or a comparison drug. Children received the active ingredient and a placebo corresponding to the comparison drug.
The clinical response (cure, improvement, no effect) was assessed at the end of therapy (days 12-16) and in a cure test (days 28-32). Safety was assessed throughout the trial for all patients on treatment. For the 321 patients evaluated at the end of treatment, the clinical success rate (cure plus improvement) was 87% for patients receiving azithromycin. For the 305 patients evaluated in the cure test, the clinical success rate was 75% for patients receiving azithromycin.
In the safety analysis, the incidence of treatment-related adverse events, primarily gastrointestinal, was 16.8% when azithromycin was taken. The most common adverse effects when taking azithromycin were diarrhea (6.4%), vomiting (4%), rash (1.7%), and nausea (1.7%).
Protocol 6. In a noncomparative clinical and microbiological study, 248 patients aged 6 months to 12 years with confirmed acute otitis media received a single oral dose of azithromycin (30 mg/kg on day 1).
The clinical success rate (i.e., cure plus improvement) at day 10 was 89% for 240 patients evaluated with the modified Intent-to-Treat (MITT) clinical analysis, and the clinical success rate (cure) was 85% for 242 patients evaluated at days 24-28.
The presumptive bacteriological eradication rates, when evaluated at days 10 and 24-28, were as follows: S. pneumoniae 70/76 (92%) and 67/76 (88%), H. influenzae 30/42 (71%) and 28/44 (64%), M. catarrhalis 10/10 (100%) and 10/10 (100%), all 110/128 (86%) and 105/130 (81%).
In the safety analysis in this study, the incidence of treatment-related adverse events, primarily gastrointestinal events, in all treated patients was 12.1%. The most frequent side effects were vomiting (5.6%), diarrhea (3.2%), and abdominal pain (1.6%).
Pharyngitis/tonsillitis
In 3 double-blind controlled trials conducted in the United States, patients received azithromycin (12 mg/kg once daily for 5 days) or a comparison drug for treatment of pharyngitis caused by documented group A hemolytic streptococci (GABHS or S. pyogenes).
The bacteriological eradication for azithromycin (for patients with documented GABHS) at days 14 and 30 was 323/340 (95%) and 255/330 (77%), respectively; clinical success (cure plus improvement) was 336/343 (98%) and 310/330 (94%), respectively.
Approximately 1% of S. pyogenes strains sensitive to azithromycin were resistant to azithromycin after therapy.
The incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated with azithromycin was 18%; the most frequent side effects were diarrhea/ liquid stool (6%), vomiting (6%), and abdominal pain (3%).
Adults
Acute bacterial exacerbations of chronic obstructive pulmonary disease
. In a randomized, double-blind, controlled clinical trial of acute exacerbations of chronic bronchitis (n=304), patients were treated with azithromycin (500 mg once daily for 3 days) or a comparison drug.
The primary endpoint of this study was clinical cure at day 21-24. For patients assessed using the clinical modified intention-to-treat analysis at day 21-24, the clinical cure rate after 3 days of azithromycin was 85% (125/147).
The bacteriologic rates of clinical cure in patients (with 3-day azithromycin therapy) at visits on days 21-24 for pathogens were: S. pneumoniae 29/32 (91%), H. influenzae 12/14 (86%), M. catarrhalis 11/12 (92%).
In the safety analysis in this study, the incidence of treatment-related adverse events, primarily gastrointestinal, in patients receiving azithromycin was 25%. The most frequent side effects were diarrhea, nausea and abdominal pain with a frequency for each symptom of 5-9%.
Acute bacterial sinusitis
In a randomized, double-blind, placebo-controlled clinical trial of acute bacterial sinusitis, patients (n=594) received azithromycin (500 mg once daily for 3 days) or another antibacterial drug. Clinical response was assessed on days 10 and 28.
The primary endpoint of this study was prospectively defined as clinical cure on day 28. For patients who were evaluated using the clinical modified intention-to-treat analysis, the clinical cure rate with 3-day treatment with azithromycin was 88% (268/303) at a visit on day 10. For patients assessed with the clinical modified intention-to-treat analysis at day 28, the clinical cure rate for patients receiving azithromycin on 3-day therapy was 71.5% (213/298).
In the safety analysis in this study, the overall incidence of treatment-related adverse events, primarily gastrointestinal, in patients receiving azithromycin was 31%; the most frequent side effects were diarrhea (17%) and nausea (7%).
In an open, noncomparative study in patients who required an initial transantral sinus puncture and who received 500 mg of azithromycin once daily for 3 days, the following results were obtained for clinical success rates on days 7 and 28 of the visit for pathogens when assessed by clinical modified intention-to-treat analysis: S. pneumonia 23/26 (88%) and 21/25 (84%), H. influenzae 28/32 (87%) and 24/32 (75%), M. catarrhalis 14/15 (93%) and 13/15 (87%).
The overall incidence of treatment-related adverse events in the noncomparative study when assessed by the clinical modified intention-to-treat analysis was 21% in patients receiving azithromycin at a dose of 500 mg once daily for 3 days, with diarrhea (9%), abdominal pain (4%) and nausea (3%) being the most frequent side effects.
Pharmacokinetics
After oral administration of azithromycin on an empty stomach at a single dose of 500 mg (2 tablets of 250 mg) in 36 healthy male volunteers, the values (±S.D.) of pharmacokinetic parameters were as follows: AUC0-72 = 4.3 (1.2) μg-h/mL; Cmax = 0.5 (0.2) μg/mL; Tmax = 2.2 (0.9) h.
The pharmacokinetic parameters of azithromycin in plasma in healthy young adults (18-40 years of age) with a regimen of 500 mg (2 capsules of 250 mg*) on day 1 followed by daily administration of 250 mg (1 capsule of 250 mg) from days 2 to 5 on days 1 and 5 were as follows: Cmax, 0.41 and 0.24 µg/mL, Tmax, 2.5 and 3.2 h, AUC0-24, 2.6 and 2.1 µg-h/mL, Cmin, 0.05 and 0.05 µg/mL, and urinary excretion (% of dose), 4.5 and 6.5.
The Cmin and Cmax were virtually unchanged from day 2 to day 5 of therapy.
* Azithromycin 250 mg tablets are bioequivalent to 250 mg capsules when taken on an empty stomach.
In a two-way cross-over study, 12 adult healthy volunteers (6 men, 6 women) received 1,500 mg of azithromycin in a single dose daily for 5 days (2 250 mg tablets on day 1, then 1 250 mg tablet on days 2-5) or 3 days (500 mg daily for days 1-3). Because of the limited number of serum samples on day 2 (3-day regimen) and day 2-4 (5-day regimen), the serum concentration-time profile for each subject followed a three-component model and the AUC0-∞ for the matched concentration profile was comparable between the 5-day and 3-day regimens.
The pharmacokinetic parameters (±SD) of azithromycin in serum on the 3-day dosing regimen at days 1 and 3 were as follows: Cmax was (0.44±0.22) and (0.54±0.25) μg/ml, and Cmax was (0.43±0.2) and (0.24±0.06) μg/ml in the 5-day dosing regimen on days 1 and 5. For the 3-day and 5-day regimens, serum AUC0-∞ were (17.4±6.2)* and (14.9±3.1)* µg-h/ml, respectively, and T1/2 from serum was 71.8 and 68.9 h.
* Total AUC for the entire 3-day and 5-day regimens.
The median exposure of azithromycin (AUC0-288) in mononuclear and polymorphonuclear leukocytes after the 5-day or 3-day regimen was more than 1000 and 800 times greater than in serum, respectively. One would expect that administration of the same total dose on the 5-day or 3-day regimen would provide comparable concentrations of azithromycin in mononuclear and polymorphonuclear leukocytes.
Two 250-mg tablets of azithromycin are bioequivalent to 1 500-mg tablet.
The absolute bioavailability of azithromycin in 250 mg capsules is 38%.
In a two-way cross-over study in which 12 healthy volunteers received a single dose of 500 mg azithromycin (2 tablets of 250 mg) with or without a high-fat diet, food was shown to increase Cmax by 23% but had no effect on AUC.
When azithromycin suspension was taken with food in 28 healthy male adults, food intake increased Cmax by 56%; AUC was unchanged.
The co-administration of an antacid containing aluminum and magnesium hydroxide with azithromycin capsules had no effect on the AUC of azithromycin, but the Cmax was reduced by 24%. Administration of cimetidine (800 mg) 2 h before azithromycin administration did not affect the absorption of azithromycin.
Distribution
The binding of azithromycin to serum proteins varies over a range of concentrations approaching human exposure and decreases from 51% at 0.02 µg/mL to 7% at 2 µg/mL.
After oral administration, azithromycin is widely distributed throughout the body with an apparent Vss of 31.1 L/kg. Tissue concentrations of azithromycin are higher than those in plasma or serum. The high tissue concentrations should not be interpreted as quantitatively related to clinical efficacy. The antimicrobial activity of azithromycin is pH-related and appears to decrease with decreasing pH. However, widespread distribution in tissues may be relevant to clinical activity.
The concentrations of azithromycin after a dose of 500 mg (2 caps. 250 mg) in adults reach 0.4 µg/ml in the skin (72-96 h after administration), a tissue (fluid)/plasma (serum) ratio of 35, 4 µg/ml in the lungs (72-96 h), a ratio of >100, in sputum (after 2-4 h) – 1 µg/ml, ratio 2, in sputum (after 10-12 h) – 2.9 µg/ml, ratio 30, in almond gland (after 9-18 h) – 4.5 µg/ml, ratio >100, in the amygdala gland (after 180 h) – 0.9 µg/ml, ratio >100, in the cervix (after 19 h) – 2.8 µg/ml, ratio 70.
The extensive distribution in tissues was confirmed by examination of additional tissues and fluids (bone, ejaculate, prostate, ovary, uterus, fallopian tube, stomach, liver, and gallbladder). Because data from adequate and well-controlled studies of azithromycin treatment of infections in these additional tissues and fluids are lacking, the clinical significance of these tissue concentration data is unknown.
After administration of 500 mg on day 1 and 250 mg daily for 4 days, only very low concentrations (less than 0.01 mcg/mL) in cerebrospinal fluid have been noted in noninflamed cerebrospinal fluid.
Metabolism
In vitro and in vivo studies have not been performed to assess the metabolism of azithromycin.
The decrease in plasma concentrations of azithromycin after single oral and intravenous administration at a dose of 500 mg is multiphasic with a mean apparent clearance from plasma of 630 ml/min and a final T1/2 of 68 h. The prolonged final T1/2 is thought to be due to extensive absorption and subsequent release from the tissues.
The excretion of azithromycin with bile, mostly unchanged, is the main route of elimination. Within 1 week, approximately 6% of the administered dose is excreted unchanged in the urine.
Dependence of pharmacokinetic parameters on some factors
Renal insufficiency. The pharmacokinetics of azithromycin have been studied in 42 adults (21 to 85 years) with varying degrees of renal impairment. After oral administration of a single dose of azithromycin 1000 mg mean Cmax and AUC0-120 were increased by 5.1 and 4.2% respectively in patients with mild and moderate renal impairment (FFR from 10 to 80 ml/min) compared with these values in subjects with normal renal function (FFR >80 ml/min). Mean Cmax and AUC values increased by 61% and 35%, respectively, in subjects with severe renal impairment (GFR <10 ml/min) compared to those with normal renal function (GFR >80 ml/min).
Hepatic failure. The pharmacokinetics of azithromycin in patients with hepatic impairment have not been established.
Gender. There are no significant differences in pharmacokinetics of azithromycin in men and women. Adjustment of the dose according to gender is not recommended.
Elderly age. In a study in healthy elderly volunteers aged 65 to 85 years, the pharmacokinetic parameters of azithromycin in elderly men were similar to those in younger volunteers; however, in elderly women, although higher peak concentrations (30-50% increase) were observed, no significant cumulation was observed. Dose adjustments based on age are not recommended.
Pediatric patients. In two clinical trials, azithromycin as an oral suspension at a dose of 10 mg/kg on day 1 followed by a dose of 5 mg/kg on days 2 through 5 was given to 2 groups of pediatric patients (ages 1-5 years and 5-15 years, respectively). Pharmacokinetic values on day 5 were as follows: Cmax = 0.216 μg/mL, Tmax = 1.9 h, and AUC0-24 = 1.822 μg-h/mL in the 1 to 5-year-old group and were Cmax = 0.383 μg/mL, Tmax = 2.4 h, and AUC0-24 = 3.109 μg-h/mL in the 5-15-year-old patient group.
Two clinical studies were conducted with 68 pediatric patients aged 3 to 16 years to determine the pharmacokinetics and safety of azithromycin as an oral suspension. Azithromycin was taken after a low-fat breakfast.
In the first study, 35 pediatric patients received azithromycin at a dose of 20 mg/kg/day (maximum daily dose of 500 mg) for 3 days, and pharmacokinetic parameters were evaluated in 34 patients.
In a second study, 33 pediatric patients received azithromycin at doses of 12 mg/kg/day (maximum daily dose of 500 mg) for 5 days; pharmacokinetics were evaluated in 31 patients.
In both studies, azithromycin concentrations were determined within 24 hours of the last daily dose. Patients with a body weight greater than 25 kg in the 3-day study and 41.7 kg in the 5-day study received a maximum daily adult dose of 500 mg. Eleven patients (25 kg or less) in the first study and 17 patients (41.7 kg or less) in the second study received a total dose of 60 mg/kg. The values of pharmacokinetic parameters (±SD) in subgroups of pediatric patients who received a total dose of 60 mg/kg in the first (n=11) and second (n=17) study were as follows: Cmax, (1.1±0.4) and (0.5±0.4) μg/mL, Tmax, (2.7±1.9) and (2.2±0.8) h, AUC0-24, (7.9±2.9) and (3.9±1.9) μg-h/mL.
The similarity of total exposure (AUC0-∞) between the 3-day and 5-day regimens in pediatric patients is unknown.
The pharmacokinetics of single dose in pediatric patients at doses of 30 mg/kg have not been studied.
Indications
Indications
According to the FDA (2020), oral azithromycin is indicated for the treatment of patients with mild to moderate infections (pneumonia: see Precautions) caused by susceptible strains of these organisms under certain conditions listed below.
Adults
Acute bacterial exacerbations of chronic obstructive pulmonary disease caused by Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Acute bacterial sinusitis caused by Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Community-acquired pneumonia caused by Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, or Streptococcus pneumoniae in patients prescribed oral therapy.
Azithromycin should not be used in patients with pneumonia who are not candidates for oral therapy because of moderate to severe disease or the presence of risk factors, such as patients with cystic fibrosis, patients with hospital-acquired infections, patients with known or suspected bacteremia, patients requiring hospitalization, elderly or debilitated patients, patients with serious underlying health problems that may interfere with the ability to respond to disease (including immunodeficiency or functional asplenia).
Pharyngitis/tonsillitis due to Streptococcus pyogenes is an alternative to first-line therapy in patients for whom first-line therapy cannot be used.
Note. Penicillin administered intramuscularly is usually the drug of choice for the treatment of infections caused by Streptococcus pyogenes and the prevention of rheumatic fever. Azithromycin is often effective in eradicating susceptible strains of Streptococcus pyogenes from the nasopharynx. Because some strains are resistant to azithromycin, azithromycin susceptibility testing should be performed during treatment of patients. There are no data confirming the effectiveness of azithromycin in the subsequent prevention of rheumatic fever.
Uncomplicated infections of the skin and skin structures caused by Staphylococcus aureus, Streptococcus pyogenes or Streptococcus agalactiae. Abscesses usually require surgery.
Urethritis and cervicitis caused by Chlamydia trachomatis or Neisseria gonorrhoeae.
An infectious disease of the genital organs in men caused by Haemophilus ducreyi (chancroid). Due to the small number of women included in clinical trials, the effectiveness of azithromycin in the treatment of chancroid in women has not been established.
Azithromycin should not be relied upon at the recommended dose to treat syphilis. Antimicrobial agents used in high doses for short periods of time to treat nongonococcal urethritis may mask or delay the onset of symptoms during the incubation period of syphilis. All patients with urethritis or cervicitis due to sexually transmitted infection should undergo serological testing for syphilis and appropriate cultures for gonorrhea at the time of diagnosis. If infection is confirmed, appropriate antimicrobial therapy should be initiated and subsequent testing for these diseases should be performed.
Before starting treatment, appropriate tests should be performed to determine the pathogen and its sensitivity to azithromycin. Azithromycin therapy may be initiated before the results of these tests are known; once results are available, antimicrobial therapy should be adjusted.
To prevent the development of drug-resistant bacteria and maintain the effectiveness of azithromycin and other antibacterial drugs, azithromycin should be used only to treat or prevent infections that are proven or reasonably suspected to be caused by susceptible bacteria. When culture and susceptibility information is available, it should be considered when selecting or changing antibiotic therapy. In the absence of such data, local epidemiology and susceptibility patterns may guide empirical selection of therapy.
Pediatric patients
Acute otitis media caused by Haemophilus influenzae, Moraxella catarrhalis or Streptococcus pneumoniae.
Community-acquired pneumonia caused by Chlamydophila pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae or Streptococcus pneumoniae in patients for whom oral therapy is indicated.
Azithromycin should not be used in pediatric patients with pneumonia who are not candidates for oral therapy because of moderate to severe disease or the presence of risk factors, such as patients with cystic fibrosis, patients with hospital-acquired infections, patients with known or suspected bacteremia, patients requiring hospitalization, patients with serious underlying health problems that may interfere with the ability to respond to disease (including immunodeficiency or functional asplenia).
Pharyngitis/tonsillitis due to Streptococcus pyogenes is an alternative to first-line therapy in patients for whom first-line therapy cannot be used.
Note. Penicillin administered intramuscularly is usually the drug of choice for the treatment of infections caused by Streptococcus pyogenes and the prevention of rheumatic fever. Azithromycin is often effective in eradicating susceptible strains of Streptococcus pyogenes from the nasopharynx. Because some strains are resistant to azithromycin, azithromycin susceptibility testing should be performed during treatment of patients. There are no data confirming the effectiveness of azithromycin in the subsequent prevention of rheumatic fever.
Before starting treatment, appropriate tests should be performed to determine the pathogen and its sensitivity to azithromycin. Azithromycin therapy may be initiated before the results of these tests are known; once results are available, antimicrobial therapy should be adjusted.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: antibiotic-azalide
ATX code: [J01FA10]
Pharmacological properties
Pharmacodynamics
Mechanism of action
Azithromycin binds to the 23S rRNA of the 50S ribosomal subunit of sensitive microorganisms. Blocks protein synthesis by inhibiting the transpeptidation/translocation stage and inhibiting the assembly of the 50S ribosomal subunit.
Under in vitro conditions, azithromycin is concentrated in phagocytes and fibroblasts. The ratio of intracellular to extracellular concentration is >30 after 1 hour of incubation. In vivo studies suggest that concentration in phagocytes may promote dissemination to inflamed tissues.
Mechanism of resistance
The most common mechanism of azithromycin resistance is modification of the 23S rRNA at positions corresponding to A2058 and A2059 (in the Escherichia coli numbering system). In addition to cross-resistance with other macrolides (erythromycin and clarithromycin), ribosomal modification can determine resistance to other classes of antibiotics (lincosamides and streptogramin B) that bind to overlapping regions of the ribosome.
Azithromycin is active against most strains of the following bacteria, both in vitro and in clinical infections: gram-positive bacteria – Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pneumoniae, Streptococcus pyogenes; gram-negative bacteria – Haemophilus ducreyi, Haemophilus influenzae, Moraxella catarrhalis, Neisseria gonorrhoeae; other bacteria – Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae.
Azithromycin exhibits in vitro minimum inhibitory concentrations (MICs) of ≤4 μg/ml against most (≥90%) strains of the following bacteria; however, the safety and effectiveness of azithromycin in the treatment of clinical infections caused by these bacteria have not been established in adequate and well-controlled studies.
Gram-positive bacteria – beta-hemolytic Streptococci (Groups C, F, G), Viridans group streptococci; gram-negative bacteria – Bordetella pertussis; anaerobic bacteria – Peptostreptococcus species, Prevotella bivia; other bacteria – Ureaplasma urealyticum, Legionella pneumophila.
Preclinical toxicology
Carcinogenicity, mutagenicity, effect on fertility. Long-term animal studies have not been conducted to evaluate the carcinogenic effects of azithromycin.
Azithromycin did not show mutagenicity in standard laboratory tests: a test on mouse lymphoma cells, micronucleus tests on human lymphocytes and mouse bone marrow.
There was no evidence of fertility impairment associated with azithromycin.
Toxicology in animals
Phospholipidosis (intracellular accumulation of phospholipids) has been observed in some tissues of mice, rats and dogs receiving repeated doses of azithromycin. This has been demonstrated in multiple organ systems (eg, eyes, dorsal root ganglia, liver, gallbladder, kidney, spleen, and/or pancreas) in dogs and rats receiving azithromycin at doses that, based on body surface area, were approximately equal (in dogs) to approximately 1/6 (in rats) of the recommended adult dose. This effect has been shown to be reversible when azithromycin is discontinued. Similarly, phospholipidosis has been observed in the tissues of neonatal rats and dogs receiving daily doses of azithromycin for 10 to 30 days. Based on pharmacokinetic data, phospholipidosis was observed in rats (30 mg/kg dose) with an observed plasma Cmax of 1.3 mcg/mL (6 times the observed Cmax of 0.216 mcg/mL in pediatric patients at a 10 mg/kg dose). Similar data were obtained in dogs (10 mg/kg dose) with an observed serum Cmax of 1.5 μg/ml (7 times the observed Cmax at the dose used in the pediatric population). When converted to body surface area in mg/m2, a 30 mg/kg dose in neonatal rats (135 mg/m2) and a 10 mg/kg dose in neonatal dogs (79 mg/m2) are approximately 0.5 and 0.3, respectively, of the recommended dose in pediatric patients with an average body weight of 25 kg. Phospholipidosis, similar to that observed in adult animals, is reversible after cessation of treatment with azithromycin. The significance of these results for animals and humans is unknown.
Electrophysiology of the heart
QTc prolongation was studied in a randomized, placebo-controlled, parallel-group study of 116 healthy volunteers who received chloroquine (1000 mg) alone or in combination with oral azithromycin (500, 1000, and 1500 mg once daily). Concomitant use with azithromycin increased the QTc interval in a dose- and concentration-dependent manner. Compared with chloroquine alone, the maximum mean (95% upper CI) increase in QTcF was 5 (10), 7 (12) and 9 (14) ms with co-administration of 500, 1000 and 1500 mg azithromycin, respectively.
Clinical studies
PEDIATRIC PATIENTS
Outcome assessments in pediatric clinical studies were conducted on days 11–14 due to the increased T1/2 of azithromycin. Data from days 11 to 14 are provided for clinical guidance. Assessments at days 24–32 were considered the primary cure endpoint.
Acute otitis media
Safety and effectiveness of azithromycin at a dose of 30 mg/kg for 5 days
Protocol 1: In a double-blind, controlled clinical trial of acute otitis media conducted in the United States, patients received azithromycin (10 mg/kg on day 1, then 5 mg/kg on days 2–5) or another antibacterial drug. For patients who completed the clinical efficacy evaluation (n=553), the clinical success rate (ie, cure + improvement) at the 11th day visit was 88% for azithromycin. For patients assessed at the 30-day visit (n=521), the clinical success rate for patients receiving azithromycin was 73%.
The safety analysis of this study found that the incidence of treatment-related adverse events, primarily gastrointestinal, in patients receiving azithromycin was 9%. The most common side effects were diarrhea/loose stools (4%), vomiting (2%), and abdominal pain (2%).
Protocol 2: In a single-arm clinical and microbiological study conducted in the United States, significant levels of beta-lactamase-producing organisms (35%) were detected and clinical efficacy was assessed in 131 patients. The combined clinical success rate (ie, cure and improvement) at the 11th day visit was 84% for patients receiving azithromycin. For the 122 patients who were assessed at the 30-day visit, the clinical success rate for patients receiving azithromycin was 70%.
Microbiological assessment was performed at the pre-treatment visit. Repeated microbiological assessments were not performed at subsequent visits.
Estimated bacterial/clinical cure rates (ie, clinical success) obtained in the evaluable group at days 11 and 30 were as follows: S. pneumoniae – 61/74 (82%) and 40/56 (71%), H. influenzae – 43/54 (80%) and 30/47 (64%), M. catarrhalis – 28/35 (80%) and 19/26 (73%), S. pyogenes – 11/11 (100%) and 7/7, all – 177/217 (82%) and 97/137 (73%).
The safety analysis in this study showed that the incidence of treatment-related adverse events, primarily gastrointestinal, was 9% in all patients treated. The most common side effect was diarrhea (4%).
Protocol 3: In another controlled comparative clinical and microbiological study conducted in the United States, azithromycin was used to treat otitis media. This study shared 2 investigators with the other trial (Protocol 2), and these 2 investigators enrolled 90% of the patients in Protocol 3. For this reason, Protocol 3 was not considered an independent study. Significant numbers of beta-lactamase-producing organisms (20%) were detected. Ninety-two (92) patients were evaluated for clinical and microbiological efficacy. The combined clinical success rate (ie, cure and improvement) in patients with the underlying pathogen at the 11th day visit for patients receiving azithromycin was 88%, and at the 30th day was 82%.
Microbiological assessment was performed at the pre-treatment visit. Repeated microbiological assessments were not performed at subsequent visits.
Estimated bacterial/clinical cure rates (i.e. clinical success) obtained in the evaluated group on days 11 and 30 were as follows: S. pneumoniae – 25/29 (86%) and 22/28 (79%), H. influenzae – 9/11 (82%) and 8/10 (80%), M. catarrhalis – 7/7 and 5/5, S. pyogenes – 2/2 and 2/2, all – 43/49 (88%) and 37/45 (82%). In the safety analysis of the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, was 4% in all patients receiving azithromycin. The most common side effect was diarrhea/loose stools (2%).
Safety and effectiveness of azithromycin at a dose of 30 mg/kg for 3 days
Protocol 4: In a double-blind, controlled, randomized, comparative clinical trial (n=366) of acute otitis media in pediatric patients aged 6 months to 12 years, patients received azithromycin (10 mg/kg/day for 3 days), a comparator drug, and placebo.
In 366 patients assessed for clinical efficacy at the 12-day visit, the clinical success rate (ie, cure plus improvement) for azithromycin was 83%. For 362 patients who were assessed on days 24–28, the clinical success rate for azithromycin was 74%.
In the safety analysis of the above study, the incidence of treatment-related adverse events, primarily gastrointestinal, in all patients receiving azithromycin was 10.6%. The most common side effects when taking azithromycin were diarrhea/loose stools (5.9%) and vomiting (2.1%).
Safety and effectiveness of a single dose of azithromycin at a dose of 30 mg/kg
Protocol 5. A double-blind, controlled, randomized study was conducted at 9 clinical sites. Pediatric patients aged 6 months to 12 years were randomized 1:1 to receive either azithromycin (a single dose of 30 mg/kg on day 1) or a comparator. Children received the active substance and a placebo corresponding to the comparator drug.
Clinical response (cure, improvement, no effect) was assessed at the end of therapy (days 12–16) and test of cure (days 28–32). Safety was assessed throughout the trial for all patients treated. For the 321 patients assessed at end of treatment, the clinical success rate (cure plus improvement) was 87% for patients receiving azithromycin. For the 305 patients who were evaluated in the test of cure, the clinical success rate was 75% for patients receiving azithromycin.
In a safety analysis, the incidence of treatment-related adverse events, primarily from the gastrointestinal tract, was 16.8% when taking azithromycin. The most common side effects with azithromycin were diarrhea (6.4%), vomiting (4%), rash (1.7%) and nausea (1.7%).
Protocol 6: In a single-arm clinical and microbiological study, 248 patients aged 6 months to 12 years with documented acute otitis media received a single oral dose of azithromycin (30 mg/kg on day 1).
For the 240 patients assessed using a clinical modified intent-to-treat (MITT) analysis, the clinical success rate (ie, cure plus improvement) at day 10 was 89%; for the 242 patients assessed at days 24–28, the clinical success rate (cure) was 85%.
Estimated bacteriological eradication rates assessed on days 10 and 24–28 were as follows: S. pneumoniae – 70/76 (92%) and 67/76 (88%), H. influenzae – 30/42 (71%) and 28/44 (64%), M. catarrhalis – 10/10 (100%) and 10/10 (100%), all – 110/128 (86%) and 105/130 (81%).
In the safety analysis in this study, the incidence of treatment-related adverse events, primarily gastrointestinal, was 12.1% in all treated patients. The most common side effects were vomiting (5.6%), diarrhea (3.2%) and abdominal pain (1.6%).
Pharyngitis/tonsillitis
In 3 double-blind, controlled studies conducted in the United States, patients received azithromycin (12 mg/kg once daily for 5 days) or a comparator for the treatment of pharyngitis caused by documented group A hemolytic streptococci (GABHS or S. pyogenes).
Bacteriologic eradication rates for azithromycin (for patients with documented GABHS) at days 14 and 30 were 323/340 (95%) and 255/330 (77%), respectively; clinical success (cure plus improvement) – 336/343 (98%) and 310/330 (94%), respectively.
Approximately 1% of S. pyogenes strains sensitive to azithromycin were resistant to azithromycin after therapy.
The incidence of treatment-related adverse events, primarily gastrointestinal, in all patients treated with azithromycin was 18%; the most common side effects were diarrhea/loose stools (6%), vomiting (6%) and abdominal pain (3%).
ADULTS
Acute bacterial exacerbations of chronic obstructive pulmonary disease
In a randomized, double-blind, controlled clinical trial of acute exacerbation of chronic bronchitis (n=304), patients were treated with azithromycin (500 mg once daily for 3 days) or a comparator drug.
The primary endpoint of this study was clinical cure rate at days 21–24. For patients assessed using a clinical modified intention-to-treat analysis on days 21–24, the clinical cure rate after 3 days of azithromycin was 85% (125/147).
Bacteriological indicators of clinical cure of patients (with 3-day therapy with azithromycin) when visiting on days 21–24 for pathogens: S. pneumoniae – 29/32 (91%), H. influenzae – 12/14 (86%), M. catarrhalis – 11/12 (92%).
In the safety analysis in this study, the incidence of treatment-related adverse events, primarily gastrointestinal, in patients receiving azithromycin was 25%. The most common side effects were diarrhea, nausea, and abdominal pain, with an incidence of 5–9% for each symptom.
Acute bacterial sinusitis
In a randomized, double-blind, double-placebo-controlled clinical trial of acute bacterial sinusitis, patients (n=594) received azithromycin (500 mg once daily for 3 days) or another antibacterial drug. Clinical response was assessed on days 10 and 28.
The primary endpoint of this study was prospectively defined as clinical cure rate at day 28. For patients assessed using a clinical modified intention-to-treat analysis, at the day 10 visit, the clinical cure rate with 3 days of azithromycin treatment was 88% (268/303). For patients assessed using a clinical modified intention-to-treat analysis at day 28, the clinical cure rate for patients receiving azithromycin with 3-day therapy was 71.5% (213/298).
In the safety analysis in this study, the overall incidence of treatment-related adverse events, primarily gastrointestinal, in patients receiving azithromycin was 31%; the most common side effects were diarrhea (17%) and nausea (7%).
In an open-label, single-arm study of patients who required an initial transantral sinus puncture and were treated with azithromycin 500 mg once daily for 3 days, when assessed using a clinical modified intention-to-treat analysis, the following results were obtained for clinical success rates at day 7 and day 28 pathogen visits: S. pneumonia 23/26 (88%) and 21/25 (84%), H. influenzae – 28/32 (87%) and 24/32 (75%), M. catarrhalis – 14/15 (93%) and 13/15 (87%).
The overall incidence of treatment-related adverse events in the single-arm study, as assessed by a modified clinical intention-to-treat analysis, was 21% in patients receiving azithromycin 500 mg once daily for 3 days, with the most common adverse events being diarrhea (9%), abdominal pain (4%), and nausea (3%).
Pharmacokinetics
After oral administration of azithromycin on an empty stomach in a single dose of 500 mg (2 tablets of 250 mg each) in 36 healthy male volunteers, the values (±S.D.) of pharmacokinetic parameters were as follows: AUC0–72 = 4.3 (1.2) μg·h / ml; Cmax = 0.5 (0.2) µg/ml; Tmax = 2.2 (0.9) h.
Pharmacokinetic parameters of azithromycin in blood plasma in healthy young people (18–40 years old) with a dosage regimen of 500 mg (2 capsules of 250 mg*) on day 1 followed by daily administration of 250 mg (1 capsule of 250 mg) from days 2 to 5 on days 1 and 5 were as follows: Cmax – 0.41 and 0.24 µg/ml, Tmax – 2.5 and 3.2 hours, AUC0-24 – 2.6 and 2.1 µg h /ml, Cmin – 0.05 and 0.05 µg/ml, urinary excretion (% of dose) – 4.5 and 6.5.
Cmin and Cmax remained virtually unchanged from the 2nd to the 5th day of therapy.
* Azithromycin 250 mg tablets are bioequivalent to 250 mg capsules when taken on an empty stomach.
In a two-way crossover study, 12 healthy adult volunteers (6 men, 6 women) received 1500 mg of azithromycin as a single dose daily for 5 days (2 tablets of 250 mg on day 1, then 1 tablet of 250 mg on days 2–5) or 3 days (500 mg daily for days 1–3). Due to the limited number of serum samples on day 2 (3-day regimen) and days 2–4 (5-day regimen), the serum concentration-time profile for each subject was fit to a three-compartment model and the AUC0–∞ for the fitted concentration profile was comparable between the 5-day and 3-day regimens.
Pharmacokinetic parameters (±SD) of azithromycin in blood serum with a 3-day dosing regimen on days 1 and 3 were as follows: Cmax – (0.44 ± 0.22) and (0.54 ± 0.25) mcg/ml, with a 5-day dosing regimen on days 1 and 5 – (0.43 ± 0.2) and (0.24±0.06) µg/ml. With a 3- and 5-day regimen, respectively, serum AUC0–∞ is (17.4±6.2)* and (14.9±3.1)* µg h/ml, T1/2 from serum is 71.8 and 68.9 hours.
* Overall AUC for the entire 3-day and 5-day regimen.
Median azithromycin exposure (AUC0-288) in mononuclear and polymorphonuclear leukocytes after a 5-day or 3-day regimen was more than 1000 and 800 times greater than in serum, respectively. Administration of the same total dose over a 5-day or 3-day regimen would be expected to provide comparable concentrations of azithromycin in mononuclear and polymorphonuclear leukocytes.
Two tables azithromycin 250 mg is bioequivalent to 1 tablet. 500 mg.
Absorption
The absolute bioavailability of azithromycin in 250 mg capsules is 38%.
In a two-way crossover study in which 12 healthy volunteers received a single dose of 500 mg azithromycin (2 x 250 mg tablets) with or without a high-fat meal, the meal was shown to increase Cmax by 23% but had no effect on AUC.
When azithromycin suspension was taken with food in 28 healthy adult men, food intake increased Cmax by 56%, but AUC did not change.
Co-administration of an antacid containing aluminum magnesium hydroxide with azithromycin capsules did not affect the AUC of azithromycin, but Cmax decreased by 24%. Taking cimetidine (800 mg) 2 hours before taking azithromycin did not affect the absorption of azithromycin.
Distribution
The binding of azithromycin to serum proteins varies over a concentration range approaching human exposure and decreases from 51% at 0.02 mcg/ml to 7% at 2 mcg/ml.
After oral administration, azithromycin is widely distributed throughout the body with an apparent Vss of 31.1 L/kg. Higher concentrations of azithromycin are observed in tissues than in plasma or serum. High tissue concentrations should not be interpreted as quantitatively associated with clinical efficacy. The antimicrobial activity of azithromycin is pH related and appears to decrease with decreasing pH. However, widespread tissue distribution may have implications for clinical activity.
Concentrations of azithromycin after taking a dose of 500 mg (2 capsules of 250 mg) in adults reach 0.4 mcg/ml in the skin (72–96 hours after administration), tissue (liquid)/plasma (serum) ratio – 35, in the lungs (72–96 hours) – 4 mcg/ml, ratio >100, in sputum (after 2–4 h) – 1 µg/ml, ratio 2, in sputum (after 10-12 hours) – 2.9 µg/ml, ratio 30, in the amygdala (after 9-18 hours) – 4.5 µg/ml, ratio >100, in the amygdala (after 180 hours) – 0.9 µg/ml, ratio >100, in the cervix (after 19 hours) – 2.8 μg/ml, ratio 70.
Extensive tissue distribution was confirmed by examination of additional tissues and fluids (bone, ejaculate, prostate, ovary, uterus, fallopian tube, stomach, liver and gall bladder). Because there are no adequate and well-controlled studies of azithromycin treatment of infections in these additional tissues and fluids, the clinical significance of these tissue concentration data is unknown.
After administration of 500 mg on day 1 and 250 mg daily for 4 days, only very low concentrations (less than 0.01 μg/ml) were observed in the cerebrospinal fluid in non-inflamed meninges.
Metabolism
In vitro and in vivo studies have not been performed to evaluate the metabolism of azithromycin.
Removal
The decrease in the concentration of azithromycin in plasma after a single oral and intravenous dose of 500 mg is multiphasic in nature with an average apparent clearance from plasma of 630 ml/min and a final T1/2 of 68 hours. It is believed that the prolonged final T1/2 is due to extensive absorption and subsequent release from tissues.
Excretion of azithromycin into bile, predominantly unchanged, is the main route of elimination. Within 1 week, approximately 6% of the administered dose is excreted unchanged in the urine.
Dependence of pharmacokinetic parameters on certain factors
Kidney failure. The pharmacokinetics of azithromycin were studied in 42 adults (21 to 85 years) with varying degrees of renal impairment. Following oral administration of a single 1000 mg dose of azithromycin, mean Cmax and AUC0–120 increased by 5.1% and 4.2%, respectively, in patients with mild to moderate renal impairment (GFR 10 to 80 mL/min) compared to those with normal renal function (GFR >80 mL/min). Mean Cmax and AUC values increased by 61% and 35%, respectively, in people with severe renal impairment (GFR 80 mL/min).
Liver failure. The pharmacokinetics of azithromycin in patients with impaired liver function has not been established.
Floor. There are no significant differences in the pharmacokinetics of azithromycin in men and women. Dose adjustment based on gender is not recommended.
Old age. When studied in healthy elderly volunteers aged 65 to 85 years, the pharmacokinetic parameters of azithromycin in elderly men were similar to those in younger volunteers; however, in older women, although higher peak concentrations were observed (30–50% increase), significant accumulation was not observed. Dose adjustment based on age is not recommended.
Pediatric patients. In two clinical studies, azithromycin oral suspension at a dose of 10 mg/kg on day 1 followed by 5 mg/kg on days 2 to 5 was given to 2 groups of pediatric patients (aged 1–5 years and 5–15 years, respectively). The values of pharmacokinetic parameters on the 5th day were as follows: Cmax = 0.216 μg/ml, Tmax = 1.9 h and AUC0–24 = 1.822 μg·h/ml in the group of children from 1 to 5 years old and were Cmax = 0.383 μg/ml, Tmax = 2.4 h and AUC0–24 = 3.109 μg·h/ml in the group patients 5–15 years old.
Two clinical studies were conducted in 68 pediatric patients aged 3 to 16 years to determine the pharmacokinetics and safety of azithromycin oral suspension. Azithromycin was taken after a low-fat breakfast.
In the first study, 35 pediatric patients received azithromycin at a dose of 20 mg/kg/day (maximum daily dose 500 mg) for 3 days, and pharmacokinetic parameters were assessed in 34 patients.
In the second study, 33 pediatric patients received azithromycin at doses of 12 mg/kg/day (maximum daily dose 500 mg) for 5 days, and pharmacokinetics were assessed in 31 patients.
In both studies, azithromycin concentrations were determined within 24 hours of the last daily dose. Patients weighing more than 25 kg in the 3-day study and 41.7 kg in the 5-day study received a maximum daily adult dose of 500 mg. Eleven patients (body weight 25 kg or less) in the first study and 17 patients (body weight 41.7 kg or less) in the second study received a total dose of 60 mg/kg. The values of pharmacokinetic parameters (±SD) in the subgroups of pediatric patients who received a total dose of 60 mg/kg in the first (n=11) and second (n=17) studies were as follows: Cmax – (1.1±0.4) and (0.5±0.4) mcg/ml, Tmax – (2.7±1.9) and (2.2±0.8) h, AUC0-24 – (7.9±2.9) and (3.9±1.9) µg·h/ml.
The similarity in total exposure (AUC0–∞) between the 3-day and 5-day regimens in pediatric patients is unknown.
Single dose pharmacokinetics in pediatric patients at doses of 30 mg/kg have not been studied.
Special instructions
Special instructions
If you miss one dose of the drug, the missed dose should be taken as early as possible, and subsequent doses should be taken at intervals of 24 hours.
Azitrox® should be taken at least one hour before or two hours after taking antacids.
Azitrox® should be used with caution in patients with mild to moderate liver dysfunction due to the possibility of developing fulminant hepatitis and severe liver failure. If there are symptoms of liver dysfunction, such as rapidly increasing asthenia, jaundice, dark urine, bleeding tendency, hepatic encephalopathy, azithromycin therapy should be discontinued and a study of the functional state of the liver should be performed.
In case of impaired renal function: in patients with GFR 10-80 ml/min, no dose adjustment is required; therapy with Azitrox® should be carried out with caution under monitoring the state of renal function. As with the use of other antibacterial drugs, during therapy with Azitrox®, patients should be regularly examined for the presence of non-susceptible microorganisms and signs of the development of superinfections, including fungal ones.
The drug Azitrox® should not be used in longer courses than indicated in the instructions, since the pharmacokinetic properties of azithromycin allow us to recommend a short and simple dosage regimen.
There is no data on a possible interaction between azithromycin and ergotamine and dihydroergotamine derivatives, but due to the development of ergotism with the simultaneous use of macrolides with ergotamine and dihydroergotamine derivatives, this combination is not recommended.
With long-term use of the drug Azitrox®, the development of pseudomembranous colitis caused by Clostridium difficile, both in the form of mild diarrhea and severe colitis, is possible. If antibiotic-associated diarrhea develops while taking the drug, as well as 2 months after the end of therapy, clostridial pseudomembranous colitis should be excluded.
Do not use drugs that inhibit intestinal motility.
When treated with macrolides, including azithromycin, prolongation of cardiac repolarization and QT interval was observed, increasing the risk of developing cardiac arrhythmias, including torsade de pointes.
Caution should be exercised when using Azitrox® in patients with proarrhythmogenic factors (especially in elderly patients): with congenital or acquired prolongation of the QT interval; in patients receiving therapy with antiarrhythmic drugs of classes IA (quinidine, procainamide), III (dofetilide, amiodarone and sotalol), cisapride, terfenadine, antipsychotic drugs (pimozide), antidepressants (citalopram), fluoroquinolones (moxifloxacin and levofloxacin), with water-electrolyte balance disorders, especially in the case of hypokalemia or hypomagnesemia, with clinically significant bradycardia, cardiac arrhythmia or severe heart failure.
The use of Azitrox® may provoke the development of myasthenic syndrome or cause an exacerbation of myasthenia gravis.
Impact on the ability to drive vehicles and machinery
If adverse reactions from the central nervous system occur, patients are advised to refrain from driving vehicles and engaging in other potentially hazardous activities that require increased concentration, speed of psychomotor and motor reactions.
Active ingredient
Active ingredient
Azithromycin
Composition
Composition
Active substance:
azithromycin dihydrate (in terms of azithromycin) – 500 mg.
Excipients:
mannitol (mannitol),
corn starch,
magnesium stearate,
sodium lauryl sulfate.
Capsule composition: body and cap:
titanium dioxide (E 171),
quinoline yellow dye (E 104),
sunset yellow dye (E110),
medical gelatin.
Pregnancy
Pregnancy
The use of the drug during pregnancy is possible only if the expected benefit to the mother outweighs the potential risk to the fetus.
Descriptions of individual cases and observational studies have shown that the use of azithromycin during pregnancy does not lead to an increase in the incidence of adverse pregnancy outcomes and is not associated with the occurrence of any specific malformations in the child.
WHO recommends azithromycin as the drug of choice for the treatment of chlamydial infection in pregnant women.
If it is necessary to prescribe the drug during lactation, breastfeeding should be stopped.
Contraindications
Contraindications
Hypersensitivity to azithromycin, erythromycin, any macrolide or ketolide antibiotics; history of cholestatic jaundice/liver dysfunction associated with previous use of azithromycin.
Side Effects
Side Effects
In clinical trials, most side effects reported were mild to moderate and reversible after discontinuation of azithromycin. Potentially serious side effects such as angioedema and cholestatic jaundice have been rarely reported. Approximately 0.7% of patients (adults and children) participating in the 5-day multiple-dose clinical trials discontinued azithromycin therapy due to treatment-related side effects. In adults receiving azithromycin 500 mg/day, the discontinuation rate due to treatment-related side effects was 0.6% after 3 days of treatment. In clinical trials in pediatric patients, when 30 mg/kg was administered either as a single dose of azithromycin or for 3 days, discontinuation of azithromycin due to treatment-associated side effects was approximately 1%. Most of the side effects that led to withdrawal were related to the gastrointestinal tract, incl. nausea, vomiting, diarrhea, or abdominal pain.
Clinical trial results
Because clinical trials are conducted under a different set of conditions, the incidence of adverse reactions observed in these clinical trials may not be the same as those obtained in other clinical trials and observed in clinical practice.
Adults
Multi-dose regimens. Overall, the most common treatment-related adverse reactions in adult patients receiving multiple doses of azithromycin were gastrointestinal effects. The most commonly reported adverse reactions were diarrhea/loose stools (4–5%), nausea (3%), and abdominal pain (2–3%).
No other treatment-related adverse events with an incidence greater than 1% were observed in patients receiving multiple doses of azithromycin.
Adverse reactions that occurred with an incidence of ≤1% included the following.
From the cardiovascular system: palpitations, chest pain.
From the gastrointestinal tract: dyspepsia, flatulence, vomiting, melena, cholestatic jaundice.
From the genitourinary system: candidiasis, vaginitis, nephritis.
From the nervous system: dizziness, headache, vertigo, drowsiness.
General: fatigue.
Allergic reactions: rash, skin itching, photosensitivity, angioedema.
Single-dose 1 g regimen: Overall, the most common side effects in patients receiving a single dose of azithromycin 1 g were related to the gastrointestinal system and were reported more frequently than in patients receiving a multiple-dose regimen.
Side effects observed in patients with a single dose of azithromycin with a frequency of 1% or more included diarrhea/loose stools (7%), nausea (5%), abdominal pain (5%), vomiting (2%), dyspepsia (1%), vaginitis (1%).
2 g single dose regimen: Overall, the most common side effects in patients receiving a single 2 g dose of azithromycin were related to the gastrointestinal system.
Side effects observed in patients with a single dose of azithromycin in this study with an incidence of 1% or more included nausea (18%), diarrhea/loose stools (14%), vomiting (7%), abdominal pain (7%), vaginitis (2%), dyspepsia (1%), dizziness (1%). Most complaints were of a moderate nature.
Pediatric patients
Single and multiple dose modes. The types of adverse events in pediatric patients were comparable to those in adults, with varying rates of occurrence for dosing regimens recommended in pediatric practice.
Acute otitis media: At the recommended total dosage of 30 mg/kg, the most common treatment-related adverse effects (≥1%) were diarrhea, abdominal pain, vomiting, nausea, and rash.
The frequency of side effects, depending on the dosage regimen, for 1-, 3- and 5-day dosing regimens was: diarrhea – 4.3; 2.6 and 1.8%; abdominal pain – 1.4; 1.7 and 1.2%; vomiting – 4.9; 2.3 and 1.1%; nausea – 1, 0.4 and 0.5%; rash – 1, 0.6 and 0.4%.
Community-Acquired Pneumonia: For the recommended dosage regimen of 10 mg/kg on day 1 followed by 5 mg/kg on days 2–5, the most common treatment-related adverse events were diarrhea/loose stools (5.8%), abdominal pain (1.9%), vomiting (1.9%), nausea (1.9%), and rash (1.6%).
Pharyngitis/tonsillitis: At the recommended dosing regimen of 12 mg/kg on days 1–5, the most common treatment-related adverse events were diarrhea (5.4%), abdominal pain (3.4%), vomiting (5.6%), nausea (1.8%), rash (0.7%), headache (1.1%).
No other treatment-related adverse events occurring with an incidence of >1% were observed in pediatric practice with any treatment regimen.
Side effects observed with an incidence of ≤1% included the following.
From the cardiovascular system: chest pain.
From the gastrointestinal tract: dyspepsia, constipation, anorexia, enteritis, flatulence, gastritis, jaundice, loose stools, oral candidiasis.
From the blood and lymphatic system: anemia, leukopenia.
From the nervous system: headache (with otitis media), hyperkinesia, dizziness, agitation, nervousness, insomnia.
General: fever, facial swelling, fatigue, fungal infection, malaise, pain.
Allergic: rash and allergic reaction.
From the respiratory system: increased cough, pharyngitis, pleural effusion, rhinitis.
From the skin and its appendages: eczema, fungal dermatitis, itching, sweating, urticaria, vesiculobullous rash.
From the senses: conjunctivitis.
Post-marketing experience
Adverse reactions reported during post-marketing experience with azithromycin in adults and/or pediatric patients for which a causal relationship has not been established include the following.
Allergic reactions: arthralgia, edema, urticaria and angioedema.
From the cardiovascular system: arrhythmia, including ventricular tachycardia and hypotension. There have been case reports of QT prolongation and torsade de pointes.
From the gastrointestinal tract: anorexia, constipation, dyspepsia, flatulence, vomiting/diarrhea, rarely leading to dehydration, pseudomembranous colitis, pancreatitis, oral candidiasis, pyloric stenosis, there are rare reports of changes in the color of the tongue.
Common: asthenia, paresthesia, fatigue, malaise and anaphylaxis (rarely fatal).
From the genitourinary system: interstitial nephritis, acute renal failure and vaginitis.
From the side of hematopoiesis: thrombocytopenia.
From the liver/biliary tract: adverse reactions associated with liver dysfunction.
From the nervous system: convulsions, dizziness/vertigo, headache, drowsiness, hyperactivity, nervousness, agitation and fainting.
Mental disorders: aggressive reaction and anxiety.
From the skin and its appendages: itching, serious skin reactions, including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS syndrome.
Senses: Hearing disturbances, including hearing loss, deafness and/or tinnitus, and reports of distortion and/or loss of taste/smell.
Laboratory abnormalities
Adults
Clinically significant abnormalities (irrespective of drug-related) were reported during clinical trials with a frequency of more than 1%: decrease in Hb, hematocrit, lymphocytes, neutrophils and blood glucose, increase in serum CPK, potassium, ALT, GGT, AST, bilirubin, creatinine, blood glucose, platelet count, lymphocytes, neutrophils and eosinophils; with a frequency of less than 1%: leukopenia, neutropenia, decrease in sodium, potassium, platelet count, increase in the number of monocytes, basophils, bicarbonate, serum alkaline phosphate, bilirubin, LDH and phosphates. Most patients with elevated serum creatinine also had abnormal baseline values.
Changes in laboratory tests were reversible.
In multiple-dose clinical trials involving more than 5000 patients, 4 patients discontinued azithromycin therapy due to treatment-related liver enzyme abnormalities and 1 due to renal dysfunction.
Pediatric patients
One-, three- and five-day regimens. Laboratory data collected during comparative clinical trials using two 3-day regimens (30 or 60 mg/kg in divided doses for 3 days) or two 5-day regimens (30 or 60 mg/kg) in divided doses for 5 days) were similar for all azithromycin dosing regimens, with the most clinically significant laboratory abnormalities occurring in frequency 1–5%. Laboratory data for patients receiving 30 mg/kg as a single dose were collected in a single-center study. In this trial, absolute neutrophil counts between 500 and 1500 cells/mm3 were observed in 10 of 64 patients receiving 30 mg/kg as a single dose, 9 of 62 patients receiving 30 mg/kg for 3 days, and 8 of 63 control patients. No patient had an absolute neutrophil count <500 cells/mm3.
In a multiple-dose clinical trial involving approximately 4,700 pediatric patients, no patients had therapy discontinued due to treatment-related laboratory abnormalities.
Interaction
Interaction
Antacids (aluminum and magnesium-containing) do not affect the bioavailability of azithromycin, but reduce its maximum concentration in the blood by 30%, so the interval between their administration should be at least 1 hour before or 2 hours after taking these drugs.
When taken simultaneously with ergotamine and dihydroergotamine derivatives, their toxic effects (vasospasm, dysesthesia) may be enhanced.
When used together with indirect anticoagulants of the coumarin series (warfarin) and azithromycin (in normal doses), patients need careful monitoring of prothrombin time.
Caution should be exercised when co-administering terfenadine and azithromycin, as it has been found that concomitant use of terfenadine and macrolides can cause arrhythmia and prolongation of the QT interval. Based on this, the above complications cannot be excluded when taking terfenadine and azithromycin together.
When azithromycin and cyclosporine are used simultaneously, a dose adjustment of cyclosporine is necessary. When taking digoxin and azithromycin together, it is necessary to monitor the concentration of digoxin in the blood, since many macrolides increase the absorption of digoxin in the intestine.
The simultaneous use of azithromycin (1200 mg) and nelfinavir (750 mg 3 times a day) causes an increase in the equilibrium concentration of azithromycin in the blood plasma; no clinically significant side effects were observed and no dose adjustment of azithromycin is required when used simultaneously with nelfinavir.
When azithromycin and zidovudine are co-administered, azithromycin has little effect on the pharmacokinetics, including renal excretion, of zidovudine or its glucuronide metabolite; azithromycin weakly interacts with cytochrome P450 isoenzymes; it has not been revealed that azithromycin is involved in pharmacological interactions similar to erythromycin and other macrolides; azithromycin is not an inducer or inhibitor of cytochrome P450 isoenzymes.
When taking azithromycin and rifabutin simultaneously, in rare cases, neutropenia may develop, the mechanism of development of which, as well as the presence of a cause-and-effect relationship with taking the drug, have not been established.
Azithromycin does not affect the blood concentrations of carbamazepine, cimetidine, didanosine, efavirenz, fluconazole, indinavir, midazolam, theophylline, triazolam, trimethoprim/sulfamethoxazole, cetirizine, sildenafil, atorvastatin, rifabutin and methylprednisolone when used simultaneously.
There have been isolated case reports of rhabdomyolysis in patients taking azithromycin and statins concomitantly.
Overdose
Overdose
Symptoms: Adverse reactions observed at doses higher than recommended were similar to those observed at usual doses, in particular nausea, diarrhea and vomiting.
Treatment: if necessary, symptomatic and supportive therapy.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature of 15–25 °C
Shelf life
Shelf life
3 years.
Manufacturer
Manufacturer
Pharmstandard-Leksredstva, Russia
Additional information
| Shelf life | 3 years. |
|---|---|
| Conditions of storage | In a dry, light-protected place at 15-25 °C |
| Manufacturer | Pharmstandard-Leksredstva, Russia |
| Medication form | capsules |
| Brand | Pharmstandard-Leksredstva |
Other forms…
Related products
Buy Azitrox, 500 mg capsules 2 pcs with delivery to USA, UK, Europe and over 120 other countries.