No products in the cart.
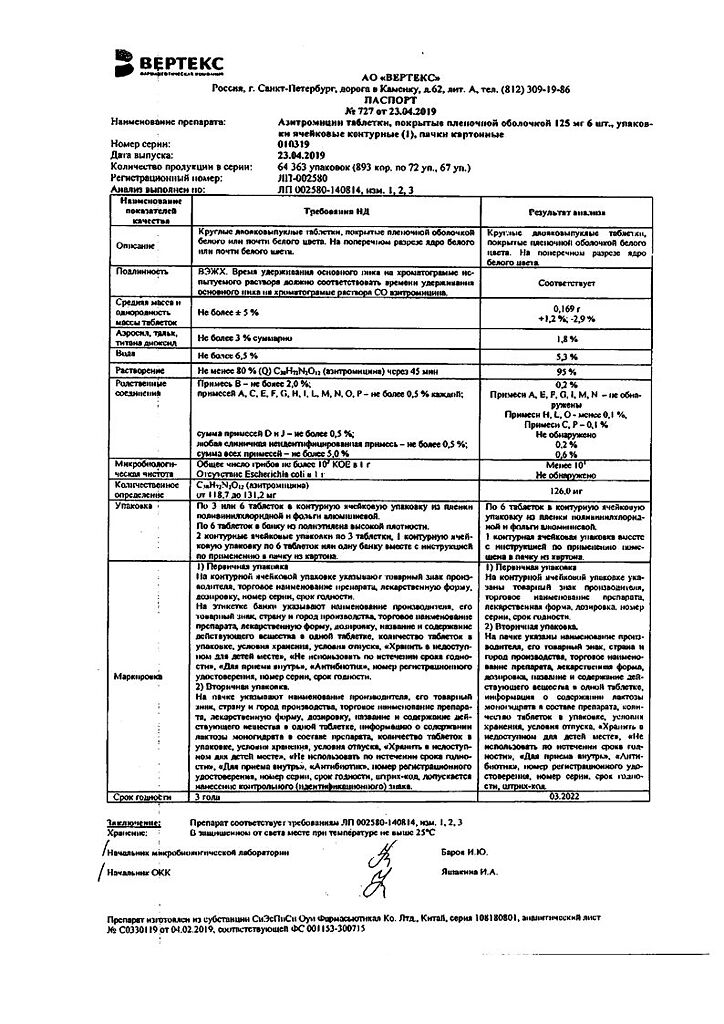
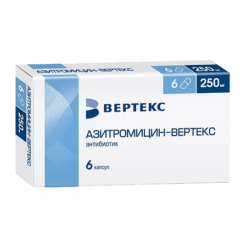
Azithromycin-Vertex, 125 mg 6 pcs
€6.41 €5.00
Out of stock
(E-mail when Stock is available)
Description
An antibiotic of the macrolide group, it is a representative of azalides. It has a wide range of antimicrobial action. The mechanism of action of azithromycin is associated with inhibition of microbial cell protein synthesis. Binding to 50S subunit of ribosomes, it inhibits peptide translocation stage, inhibits protein synthesis, slows down growth and reproduction of bacteria. It acts bacteriostatic. At high concentrations it has a bactericidal effect. Azithromycin is sensitive to Gram-positive cocci: Streptococcus pneumoniae (penicillin-sensitive strains), Streptococcus pyogenes, Staphylococcus aureus (methicillin-sensitive strains); aerobic Gram-negative bacteria: Haemophilus influenzae, Haemophilus parainfluenzae, Legionella pneumophila, Moraxella catarrhalis, Pasteurella multocida, Neisseria gonorrhoeae; some anaerobic microorganisms: Clostridium perfringens, Fusobacterium spp., Prevotella spp., Porphyriomonas spp.; and Chlamydia trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, Mycoplasma pneumoniae, Mycoplasma hominis, Borrelia burgdorferi. Microorganisms with acquired resistance to azithromycin: aerobic gram-positive microorganisms -Streptococcus pneumoniae (penicillin-resistant strains and strains with moderate sensitivity to penicillin). Microorganisms with natural resistance: aerobic gram-positive microorganisms – Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis (methicillin-resistant strains), anaerobic microorganisms – Bacteroides fragilis. Cases of cross-resistance between Streptococcus pneumoniae, Streptococcus pyogenes (group A beta-hemolytic streptococcus), Enterococcus faecalis and Staphylococcus aureus (methicillin-resistant strains) to erythromycin, azithromycin, other macrolides and Lincosamides have been described. Asithromycin has not been used to treat infections caused by Salmonella typhi (MIC < 16 mg/l) and Shigella spp. Pharmacokinetics Azithromycin is well absorbed and rapidly distributed in the body after oral administration. After a single dose of 500 mg the bioavailability is 37% due to the “first pass” effect through the liver. Cmax in blood plasma is reached after 2-3 hours and is 0.4 mg/l. Protein binding is inversely proportional to plasma concentration and is 7-50%. The apparent Vd is 31.1 l/kg. It penetrates through cell membranes (effective in infections caused by intracellular pathogens). It is transported by phagocytes to the site of infection, where it is released in the presence of bacteria. Easily penetrates the histohematic barriers and enters the tissues. Concentration in tissues and cells is 10-50 times higher than in plasma and 24-34% higher in the focus of infection than in healthy tissues. The drug is metabolized in the liver. Metabolites have no antimicrobial activity. T1/2 is very long, 35-50 h. T1/2 from tissues is much longer. The therapeutic concentration of azithromycin is maintained up to 5-7 days after the last dose. Azithromycin is excreted mainly unchanged – 50% through the intestine, 6% by the kidneys. After intravenous infusion, azithromycin rapidly penetrates from serum to tissues. Concentrated in phagocytes and without affecting their function, azithromycin migrates to the focus of inflammation, accumulating directly in infected tissues. The pharmacokinetics of azithromycin in healthy volunteers after a single IV infusion lasting more than 2 hours at a dose of 1-4 g (solution concentration 1 mg/ml) has a linear dependence and is proportional to the administered dose. In healthy volunteers, the Cmax in serum was 1.14 µg/mL when azithromycin was infused intravenously at a dose of 500 mg (solution concentration 1 mg/mL) for 3 h. Cmin in serum (0.18 µg/mL) was noted for 24 h and the AUC was 8.03 µg×mL/h. Similar pharmacokinetic values were obtained in patients with community-acquired pneumonia who received 3-hour IV infusions for 2 to 5 days. Vd is 33.3 L/kg. After administration of azithromycin daily at a dose of 500 mg (1-hour infusion duration) for 5 days, an average of 14% of the dose is excreted by the kidneys over a 24-hour dosing interval. The T1/2 is 65-72 h. The large Vd (33.3 L/kg) and high plasma clearance (10.2 ml/min/kg) suggest that the long T1/2 is a consequence of accumulation of the antibiotic in tissues followed by its slow release.
Indications
Indications
Infectious and inflammatory diseases caused by microorganisms sensitive to the drug:
– infections of the upper respiratory tract and ENT organs: pharyngitis, tonsillitis, sinusitis, otitis media;
– lower respiratory tract infections: acute bronchitis, exacerbation of chronic bronchitis, pneumonia, incl. caused by atypical pathogens;
— infections of the skin and soft tissues: acne vulgaris of moderate severity, erysipelas, impetigo, secondary infected dermatoses;
– the initial stage of Lyme disease (borreliosis) – erythema migrans;
– urinary tract infections caused by Chlamydia trachomatis (urethritis, cervicitis).
Pharmacological effect
Pharmacological effect
An antibiotic of the macrolide group, a representative of the azalides. Has a wide spectrum of antimicrobial action. The mechanism of action of azithromycin is associated with the suppression of protein synthesis in microbial cells. By binding to the 50S ribosomal subunit, it inhibits peptide translocase at the translation stage, suppresses protein synthesis, and slows down the growth and reproduction of bacteria. Acts bacteriostatically. In high concentrations it has a bactericidal effect.
It is active against a number of gram-positive, gram-negative, anaerobic, intracellular and other microorganisms.
Gram-positive cocci are sensitive to azithromycin: Streptococcus pneumoniae (penicillin-sensitive strains), Streptococcus pyogenes, Staphylococcus aureus (methicillin-sensitive strains); aerobic gram-negative bacteria: Haemophilus influenzae, Haemophilus parainfluenzae, Legionella pneumophila, Moraxella catarrhalis, Pasteurella multocida, Neisseria gonorrhoeae; some anaerobic microorganisms: Clostridium perfringens, Fusobacterium spp., Prevotella spp., Porphyriomonas spp.; as well as Chlamydia trachomatis, Chlamydia pneumoniae, Chlamydia psittaci, Mycoplasma pneumoniae, Mycoplasma hominis, Borrelia burgdorferi.
Microorganisms with acquired resistance to azithromycin: aerobic gram-positive microorganisms – Streptococcus pneumoniae (penicillin-resistant strains and strains with intermediate sensitivity to penicillin).
Microorganisms with natural resistance: aerobic gram-positive microorganisms – Enterococcus faecalis, Staphylococcus aureus, Staphylococcus epidermidis (methicillin-resistant strains), anaerobic microorganisms – Bacteroides fragilis.
Cases of cross-resistance between Streptococcus pneumoniae, Streptococcus pyogenes (group A beta-hemolytic streptococcus), Enterococcus faecalis and Staphylococcus aureus (methicillin-resistant strains) to erythromycin, azithromycin, other macrolides and lincosamides have been described.
Azithromycin has not been used to treat infections caused by Salmonella typhi (MIC < 16 mg/L) and Shigella spp.
Pharmacokinetics
After oral administration, azithromycin is well absorbed and quickly distributed in the body. After a single dose of 500 mg, bioavailability is 37% due to the “first pass” effect through the liver. Cmax in blood plasma is reached after 2-3 hours and is 0.4 mg/l.
Protein binding is inversely proportional to plasma concentration and ranges from 7-50%. The apparent Vd is 31.1 l/kg. Penetrates through cell membranes (effective against infections caused by intracellular pathogens). Transported by phagocytes to the site of infection, where it is released in the presence of bacteria. Easily penetrates histohematic barriers and enters tissues. The concentration in tissues and cells is 10-50 times higher than in plasma, and at the site of infection it is 24-34% higher than in healthy tissues.
The drug is metabolized in the liver. Metabolites do not have antimicrobial activity.
T1/2 is very long – 35-50 hours. T1/2 from fabrics is much longer. The therapeutic concentration of azithromycin lasts up to 5-7 days after taking the last dose. Azithromycin is excreted mainly unchanged – 50% through the intestines, 6% by the kidneys.
After IV infusion, azithromycin quickly penetrates from the blood serum into the tissues. Concentrating in phagocytes and without disrupting their function, azithromycin migrates to the site of inflammation, accumulating directly in infected tissues.
The pharmacokinetics of azithromycin in healthy volunteers after a single intravenous infusion lasting more than 2 hours at a dose of 1-4 g (solution concentration 1 mg/ml) has a linear relationship and is proportional to the administered dose.
In healthy volunteers, with an intravenous infusion of azithromycin at a dose of 500 mg (solution concentration 1 mg/ml) for 3 hours, Cmax in the blood serum was 1.14 μg/ml. Serum Cmin (0.18 μg/ml) was observed over 24 hours and AUC was 8.03 μg×ml/h. Similar pharmacokinetic values were obtained in patients with community-acquired pneumonia who were prescribed IV 3-hour infusions for 2 to 5 days. Vd is 33.3 l/kg.
After administration of azithromycin daily at a dose of 500 mg (infusion duration 1 hour) for 5 days, an average of 14% of the dose is excreted by the kidneys over a 24-hour dosing interval.
T1/2 is 65-72 hours. A large Vd (33.3 l/kg) and high plasma clearance (10.2 ml/min/kg) suggest that a long T1/2 is a consequence of the accumulation of the antibiotic in tissues followed by its slow release.
Special instructions
Special instructions
If you miss a dose of an antibiotic, take the missed dose as soon as possible, and subsequent doses should be taken 24 hours apart.
After discontinuation of azithromycin therapy, hypersensitivity reactions in some patients may persist for an extended period of time and may require specific therapy under medical supervision.
Impact on the ability to drive vehicles and operate machinery
Given the likelihood of developing side effects from the central nervous system, caution should be exercised when driving vehicles and operating machinery.
Active ingredient
Active ingredient
Azithromycin
Composition
Composition
1 tablet contains:
Active ingredients:
azithromycin (in the form of dihydrate) – 125 mg,
Excipients:
microcrystalline cellulose – 9 mg,
lactose monohydrate – 8.366 mg,
povidone (K-30) – 6.5 mg,
crospovidone – 6.5 mg,
sodium lauryl sulfate – 0.325 mg,
colloidal silicon dioxide – 1.65 mg,
magnesium stearate – 1.65 mg.
Film shell composition: hypromellose – 3 mg, talc – 1 mg, titanium dioxide – 0.55 mg, macrogol 4000 – 0.45 mg.
Contraindications
Contraindications
– severe liver and/or kidney failure;
– children under 12 years of age with body weight less than 45 kg (for this dosage form);
– breastfeeding;
– simultaneous use with ergotamine and dihydroergotamine;
– hypersensitivity to macrolide antibiotics.
With caution
– moderate dysfunction of the liver and kidneys;
– with arrhythmias or predisposition to arrhythmias and prolongation of the QT interval;
– with the combined use of terfenadine, warfarin, digoxin.
Side Effects
Side Effects
From the digestive system: nausea, vomiting, diarrhea, abdominal pain, loose stools, flatulence, indigestion, anorexia, constipation, discoloration of the tongue, pseudomembranous colitis, cholestatic jaundice, hepatitis, changes in laboratory parameters of liver function, liver failure, liver necrosis (possibly fatal).
Allergic reactions: itching, skin rashes, angioedema, urticaria, photosensitivity, anaphylactic reaction (in rare cases fatal), erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis.
From the cardiovascular system: palpitations, arrhythmia, ventricular tachycardia, increased QT interval, bidirectional ventricular tachycardia.
From the nervous system: dizziness/vertigo, headache, convulsions, drowsiness, paresthesia, asthenia, insomnia, hyperactivity, aggressiveness, anxiety, nervousness.
From the senses: tinnitus, reversible hearing impairment up to deafness (when taking high doses for a long time), impaired perception of taste and smell.
From the circulatory and lymphatic systems: thrombocytopenia, neutropenia, eosinophilia.
From the musculoskeletal system: arthralgia.
From the genitourinary system: interstitial nephritis, acute renal failure.
Other: vaginitis, candidiasis.
Interaction
Interaction
Antacids do not affect the bioavailability of azithromycin, but reduce the maximum concentration in the blood by 30%, so the drug should be taken at least 1 hour before or 2 hours after taking these drugs and food.
When administered parenterally, azithromycin does not affect the plasma concentrations of cimetidine, efavirenz, fluconazole, indinavir, midazolam, triazolam, co-trimoxazole (sulfamethoxazole + trimethoprim) when used together, however, the possibility of such interactions should not be excluded when azithromycin is administered orally.
If combined use with cyclosporine is necessary, it is recommended to monitor the level of cyclosporine in the blood.
When taking digoxin and azithromycin together, it is necessary to monitor the concentration of digoxin in the blood, because many macrolides increase the absorption of digoxin in the intestine, thereby increasing its concentration in the blood plasma.
If co-administration with warfarin is necessary, careful monitoring of prothrombin time is recommended.
Concomitant use of terfenadine and macrolide antibiotics causes arrhythmia and prolongation of the QT interval. Based on this, the above complications cannot be excluded when taking terfenadine and azithromycin together. Since there is a possibility of inhibition of the CYP3A4 isoenzyme by azithromycin in parenteral form when used together with cyclosporine, terfenadine, ergot alkaloids, cisapride, pimozide, quinidine, astemizole and other drugs whose metabolism occurs with the participation of this isoenzyme, the possibility of such an interaction should be taken into account when prescribing azithromycin for oral administration.
When azithromycin and zidovudine are used together, azithromycin does not affect the pharmacokinetic parameters of zidovudine in the blood plasma or the renal excretion of zidovudine and its glucuronidated metabolite. However, the concentration of the active metabolite, phosphorylated zidovudine, increases in mononuclear cells of peripheral vessels. The clinical significance of this fact is unclear.
When macrolides are taken simultaneously with ergotamines and dihydroergotamine, their toxic effects may occur.
Overdose
Overdose
Symptoms: temporary hearing loss, nausea, vomiting, diarrhea.
Treatment: symptomatic.
Storage conditions
Storage conditions
In a place protected from light, at a temperature not exceeding 25 °C.
Shelf life
Shelf life
3 years
Manufacturer
Manufacturer
Vertex, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | In a light-protected place at a temperature not exceeding 25 °C. |
| Manufacturer | Vertex, Russia |
| Medication form | pills |
| Brand | Vertex |
Other forms…
Related products
Buy Azithromycin-Vertex, 125 mg 6 pcs with delivery to USA, UK, Europe and over 120 other countries.