No products in the cart.
Avelox, 400 mg, 5 pcs.
€15.60 €13.52
Description
Pharmacological group Quinolones/fluoroquinolones
Characteristics
Antibacterial agent of IV generation fluoroquinolones. Moxifloxacin hydrochloride is a yellowish or yellow crystalline substance. It differs from other fluoroquinolones by the presence of a methoxy group in position 8 and bicycloamine in position 7 of the molecule. Molecular weight is 437.9.
Indications
Indications
According to rxlist.com (2020), oral and IV moxifloxacin is indicated for the treatment of infections caused by susceptible strains of microorganisms in adult patients (over 18 years of age).
Community-acquired pneumonia caused by Streptococcus pneumoniae (including multidrug-resistant strains [MDRSP]), Haemophilus influenzae, Moraxella catarrhalis, methicillin-sensitive Staphylococcus aureus, Klebsiella pneumoniae, Mycoplasma pneumoniae, or Chlamydia pneumoniae.
Uncomplicated infectious diseases of the skin and its appendages caused by methicillin-sensitive Staphylococcus aureus or Streptococcus pyogenes.
Complicated infectious diseases of the skin and its appendages caused by methicillin-sensitive Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae or Enterobacter cloacae.
Complicated intra-abdominal infections, including polymicrobial infections such as abscess formation caused by Escherichia coli, Bacteroides fragilis, Streptococcus anginosus, Streptococcus constellatus, Enterococcus faecalis, Proteus mirabilis, Clostridium perfringens, Bacteroides thetaiotaomicron or Peptostreptococcus spp.
Plague, including pneumonic and septicemic plague caused by Yersinia pestis, and prevention of plague. The effectiveness of moxifloxacin could not be studied in patients with plague. This indication is based on an efficacy study conducted in animals only.
Acute bacterial sinusitis caused by Streptococcus pneumoniae, Haemophilus influenzae or Moraxella catarrhalis.
Because fluoroquinolones, including moxifloxacin, are associated with serious adverse reactions (see Precautions) and acute bacterial sinusitis is a self-limiting condition in some patients, use for the treatment of acute bacterial sinusitis is limited to patients who have no alternative treatment options (see Precautions).
Exacerbation of chronic bronchitis caused by Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, methicillin-sensitive Staphylococcus aureus or Moraxella catarrhalis.
Because the use of fluoroquinolones, including moxifloxacin, is associated with the development of serious adverse reactions (see “Precautions”), and exacerbation of chronic bronchitis in some patients is a self-limiting disease, use in the treatment of exacerbation of chronic bronchitis is only possible for patients who have no alternative treatment options.
Pharmacological effect
Pharmacological effect
Pharmacological group
Quinolones/fluoroquinolones
Characteristics
IV generation fluoroquinolone antibacterial agent. Moxifloxacin hydrochloride is a yellowish or yellow crystalline substance. It differs from other fluoroquinolones by the presence in the structure of the molecule of a methoxy group at position 8 and bicycloamine at position 7. Molecular weight – 437.9.
Special instructions
Special instructions
Before starting treatment, appropriate tests should be performed to identify the microorganisms causing the disease and assess sensitivity to moxifloxacin. Therapy with moxifloxacin may be started pending the results of these tests. Once test results are known, appropriate therapy should be continued.
Active ingredient
Active ingredient
Moxifloxacin
Composition
Composition
1 tablet contains:
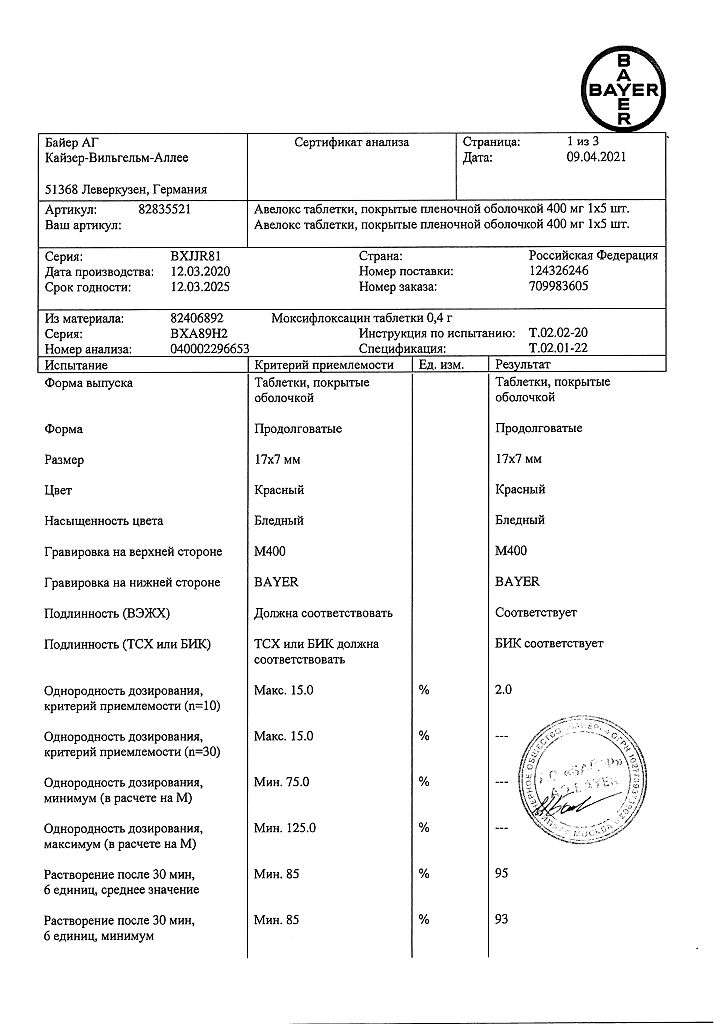
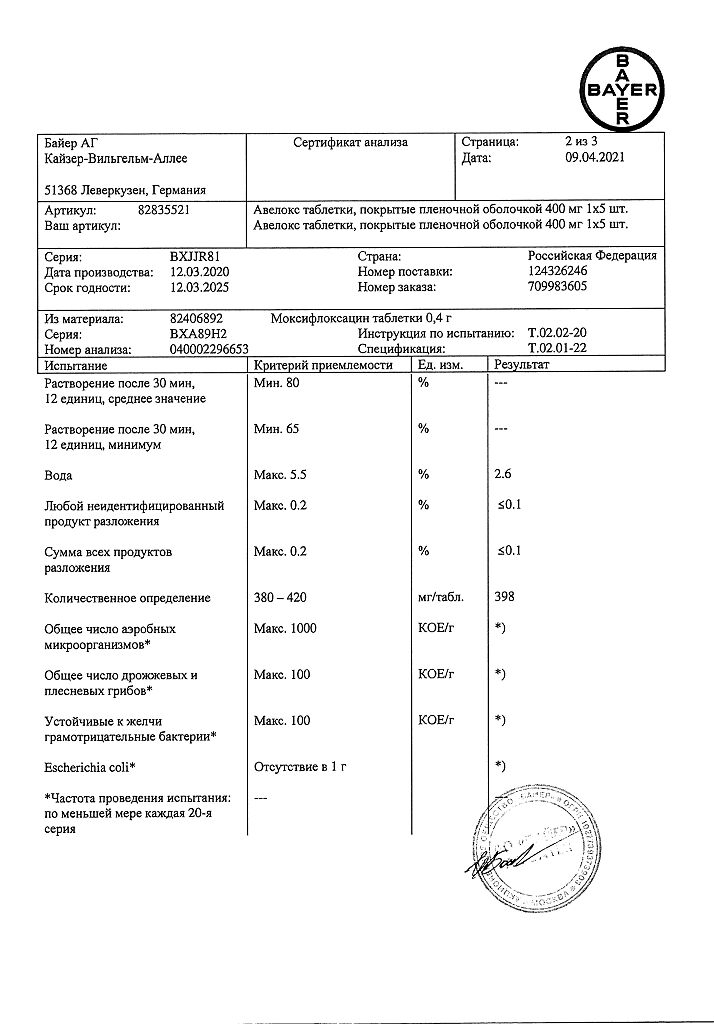
Active ingredient: moxifloxacin hydrochloride – 436.8 mg, equivalent to 400.0 mg moxifloxacin.
Excipients: microcrystalline cellulose (Avicel) – 136.0 mg, croscarmellose sodium – 32.0 mg, lactose monohydrate – 68.0 mg, magnesium stearate – 6.0 mg, shell – hypromellose – 12.6 mg, red iron oxide dye – 0.42 mg, macrogol 4000 (polyethylene glycol 4000) – 4.2 mg, titanium dioxide – 3.78 mg.
Pregnancy
Pregnancy
Use during pregnancy is possible if the expected effect of therapy outweighs the potential risk to the fetus (adequate and strictly controlled studies of the safety of use in pregnant women have not been conducted).
Teratogenic effects. Moxifloxacin was not teratogenic when administered orally to pregnant rats during organogenesis at doses above 500 mg/kg/day, corresponding to approximately 0.24 MRDI (based on AUC values), but a decrease in fetal body weight and a slight delay in skeletal formation were observed, indicating fetotoxicity. When moxifloxacin was administered intravenously to pregnant rats at a dose of 80 mg/kg/day (approximately 2 times the MRDC in terms of body surface (mg/m2), toxicity to females and minimal effects on the fetus, weight and appearance of the placenta were observed.
With intravenous administration of doses above 80 mg/kg/day, no teratogenic effect was observed. IV administration of a dose of 20 mg/kg/day to female rabbits during pregnancy during the period of organogenesis (approximately identical to MRDC when taken orally) led to a decrease in fetal body weight and a delay in skeletal ossification. Signs of toxicity to female rabbits at these doses included mortality, miscarriages, a marked decrease in food intake, decreased water intake, and hypoactivity. There was no evidence of teratogenicity when administered orally at a dose of 100 mg/kg/day (2.5 MURD) in Cynomolgus monkeys.
The incidence of weight loss in newborn pups increased at a dose of 100 mg/kg/day. In rats, it was found that when administered orally at a dose of 500 mg/kg/day, the following effects were observed: a slight increase in the duration of pregnancy, prenatal losses, decreased weight in newborn pups, and decreased neonatal survival. The toxic effect of moxifloxacin on the maternal body was manifested when a dose of 500 mg/kg/day was administered to rats during pregnancy.
FDA category of effect on the fetus is C.
Moxifloxacin is excreted into the breast milk of rats. Because moxifloxacin can pass into the breast milk of nursing women and cause serious adverse reactions in breastfed infants, nursing women should discontinue either breastfeeding or the use of moxifloxacin (given the importance of the drug for the mother).
Contraindications
Contraindications
Hypersensitivity (including to other quinolones), age under 18 years (safety and effectiveness of use have not been determined; it should be borne in mind that moxifloxacin causes arthropathy in young growing animals).
Side Effects
Side Effects
The following serious and important adverse reactions are described in detail in the Precautions section:
– disabling and potentially irreversible serious adverse reactions, including tendonitis and tendon rupture, peripheral neuropathy and effects on the central nervous system (see “Precautions”);
– tendonitis and tendon rupture (see “Precautions”);
– peripheral neuropathy (see “Precautions”);
– effect on the central nervous system (see “Precautions”);
– exacerbation of myasthenia gravis (see “Precautions”);
– prolongation of the QT interval (see “Precautions”);
– other serious and sometimes fatal reactions (see “Precautions”);
– hypersensitivity reactions (see “Precautions”);
– diarrhea associated with Clostridium difficile (see “Precautions”);
– changes in blood glucose levels (see “Precautions”);
– photosensitivity/phototoxicity (see “Precautionary measures”);
– development of drug-resistant bacteria (see “Precautions”).
Clinical trial results
Because clinical trials are conducted under a different set of conditions, the incidence of adverse reactions observed in these clinical trials may not be the same as those obtained in other clinical trials and observed in clinical practice.
The data below reflects exposure to moxifloxacin in 14,981 patients in 71 controlled phase II to IV active drug clinical trials for various indications. The mean age of patients was 50 years (approximately 73% were <65 years), 50% were male, 63% were Caucasian, 12% were Asian, and 9% were African American. Patients received moxifloxacin 400 mg once daily orally, intravenously, or sequentially (iv then orally). The duration of treatment usually ranged from 6 to 10 days, the average number of days of therapy was 9 days.
Therapy was discontinued due to side effects in 5% of patients overall, 4% of patients with oral administration, 4% with IV administration, and 8% with sequential therapy (oral and IV). The most common adverse reactions (>0.3%) leading to discontinuation of therapy when taking moxifloxacin orally were nausea, diarrhea, dizziness and vomiting. The most common side effect leading to discontinuation with IV administration was rash. The most common adverse reactions leading to discontinuation of sequential therapy (iv, orally) were diarrhea and hyperthermia.
Adverse events observed in controlled clinical trials with the active drug in patients receiving moxifloxacin (N=14981), with an incidence of >0.1%, are presented below. The most common adverse reactions (≥3%) were nausea, diarrhea, headache and dizziness.
From the nervous system and sensory organs: headache (4%), dizziness (3%), insomnia (2%); ≥0.1% <1% - fatigue, malaise, asthenia, drowsiness, tremor, lethargy, paresthesia, hypoesthesia, syncope, anxiety, confusion, agitation, depression, nervousness, restlessness, hallucinations, disorientation, vertigo, blurred vision, perversion of taste, ringing in the ears.
From the cardiovascular system and blood (hematopoiesis, hemostasis): anemia (1%); ≥0.1% <1% - atrial fibrillation, palpitations, tachycardia, angina pectoris, heart failure, cardiac arrest, bradycardia, hypertension, hypotension, thrombocythemia, eosinophilia, neutropenia, thrombocytopenia, leukopenia, leukocytosis.
From the respiratory system: ≥0.1% <1% - shortness of breath, asthma, wheezing, bronchospasm.
From the gastrointestinal tract: nausea (7%), diarrhea (6%), vomiting (2%), constipation (2%), abdominal pain (2%), dyspepsia (1%); ≥0.1% <1% - dry mouth, abdominal discomfort, flatulence, bloating, gastritis, GERD, gastroenteritis, liver dysfunction.
Metabolism: hypokalemia (1%); ≥0.1% <1% - hyperglycemia, anorexia, hyperlipidemia, decreased appetite, dehydration.
From the genitourinary system: ≥0.1% <1% - renal failure, dysuria, vaginal candidiasis, vulvovaginal itching.
From the musculoskeletal system and connective tissue: ≥0.1% <1% - pain in the back, limbs, arthralgia, muscle spasm, musculoskeletal pain.
From the skin: ≥0.1% <1% - rash, itching, hyperhidrosis, erythema, urticaria, allergic dermatitis, night sweats.
Laboratory and instrumental indicators: increased ALT level (1%); ≥0.1% <1% - increased AST level, increased GGT, increased alkaline phosphatase in the blood, prolongation of the QT interval on ECT, increased LDH in the blood, increased amylase in the blood, increased lipase, increased creatinine in the blood, increased urea in the blood, increased hematocrit, increased PT, increased eosinophil count, increased APTT, increased triglyceride levels in the blood, increased uric acid in blood.
Other: pyrexia (1%), ≥0.1% <1% - chills, extravasation at the IV injection site, including phlebitis, edema, urticaria, fungal infection, pain, incl. chest pain, facial pain.
Changes in laboratory parameters
Changes in laboratory parameters not listed above and observed in ≥2% of patients and at a frequency higher than in the control group included increases in mean corpuscular hemoglobin (MCH), neutrophils, leukocytes, PT ratio, ionized calcium, chloride, albumin, globulin, bilirubin; decrease in Hb, number of red blood cells, neutrophils, eosinophils, basophils, glucose, partial pressure of oxygen (PO2), bilirubin and amylase. It is not possible to determine whether any of the above laboratory abnormalities were caused by the drug or the underlying disease.
Post-marketing experience
Listed below are the adverse reactions that have been reported with moxifloxacin. Because reports of these cases are voluntary, from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
From the nervous system and sensory organs: impaired coordination of movements, gait disturbance (see “Precautions”), myasthenia gravis (exacerbation) (see “Precautions”), muscle weakness, peripheral neuropathy (which may be irreversible), polyneuropathy (see “Precautions”), psychotic reactions (very rarely resulting in self-injurious behavior such as suicidal thoughts/attempts) (see “Precautions”) precautions”), hearing impairment, including deafness (in most cases is reversible), loss of vision (especially with reactions from the central nervous system, which in most cases are transient).
From the cardiovascular system and blood (hematopoiesis, hemostasis): ventricular tachyarrhythmia (including very rare cases of cardiac arrest and torsade de pointes, usually in patients with concomitant severe proarrhythmic symptoms), agranulocytosis, pancytopenia (see “Precautions”).
From the respiratory system: allergic pneumonitis (see “Precautions”).
From the gastrointestinal tract: hepatitis (mainly cholestatic), liver failure (including fatal cases), jaundice, acute liver necrosis (see “Precautions”).
From the genitourinary system: interstitial nephritis (see “Precautions”).
From the musculoskeletal system and connective tissue: tendon rupture (see “Precautions”).
From the skin: photosensitivity/phototoxicity reactions (see “Precautions”), Stevens-Johnson syndrome, toxic epidermal necrolysis.
Allergic reactions: anaphylactic reactions, anaphylactic shock, angioedema (including laryngeal edema).
Interaction
Interaction
Antacids, sucralfate, multivitamins and other drugs containing polyvalent cations. Moxifloxacin forms chelate complexes with alkaline earth and transition metal cations. Taking moxifloxacin orally with antacids containing aluminum or magnesium, sucralfate, metal cations such as iron, or with multivitamins containing iron or zinc, or with drugs containing divalent and trivalent cations, such as didanosine buffered tablets for oral suspension or children’s powder for oral solution, may have significant effects. on the absorption of moxifloxacin, resulting in systemic concentrations that will be significantly lower than desired.
In a study of 12 healthy volunteers, it was shown that a single oral dose of moxifloxacin 400 mg 2 hours before, simultaneously or 4 hours after taking an aluminum/magnesium antacid (900 mg aluminum hydroxide and 600 mg magnesium hydroxide once orally) led to a decrease in the AUC value of moxifloxacin by 26, 60 and 23%, respectively.
When co-administered with moxifloxacin tablets and iron sulfate (100 mg once daily for two days), the mean AUC and Cmax values of moxifloxacin decreased by 39 and 59%, respectively.
Moxifloxacin should be taken orally at least 4 hours before or 8 hours after taking magnesium- or aluminum-containing antacids, sucralfate, metal cations such as iron, and multivitamins containing zinc.
Warfarin. In a study involving 24 healthy volunteers, no significant effect of moxifloxacin (400 mg once daily for 8 days) on the pharmacokinetics of the R- or S-isomers of warfarin was found (warfarin in a single dose of 25 mg on day 5), and no significant change in PT was noted in the presence of moxifloxacin. However, fluoroquinolones, including moxifloxacin, have been reported to enhance the anticoagulant effect of warfarin or its derivatives. In addition, the infectious disease and the accompanying inflammatory process, age and general condition of the patient are risk factors for increased anticoagulant activity. Therefore, PT, INR and other coagulation parameters should be carefully monitored in patients taking warfarin and moxifloxacin concomitantly.
Antidiabetic agents. Changes in blood glucose, including hyperglycemia and hypoglycemia, have been reported in patients receiving concomitant fluoroquinolones, including moxifloxacin, and antidiabetic agents. Therefore, careful monitoring of blood glucose levels is recommended during concurrent use. If hypoglycemia occurs, moxifloxacin should be discontinued and appropriate therapy initiated immediately.
NSAIDs. Concomitant use of NSAIDs with fluoroquinolones, including moxifloxacin, may increase the risk of CNS stimulation and seizures.
Drugs that prolong the QT interval. There is limited information about the potential PDV of moxifloxacin and other drugs that prolong the QTc interval on the ECG in humans. Sotalol, a Class III antiarrhythmic agent, further increases the QTc interval when combined with high doses of IV moxifloxacin in dogs. Therefore, the use of moxifloxacin with class IA and class III antiarrhythmic drugs should be avoided.
Calcium, digoxin, itraconazole, morphine, probenecid, ranitidine, theophylline, cyclosporine and warfarin did not significantly affect the pharmacokinetics of moxifloxacin. These results and data from in vitro studies suggest that moxifloxacin is unlikely to have a significant effect on the metabolic clearance of drugs metabolized with the participation of isoenzymes CYP3A4, CYP2D6, CYP2C9, CYP2C19, CYP1A2.
Moxifloxacin had no clinically significant effect on the pharmacokinetics of atenolol, digoxin, glyburide, itraconazole, oral contraceptives, theophylline, cyclosporine and warfarin.
Atenolol. In a crossover study in 24 healthy volunteers (12 men, 12 women), the mean AUC of atenolol following a single 50 mg oral dose and placebo was similar to that observed when atenolol was coadministered with a single 400 mg oral dose of moxifloxacin. The mean Cmax of atenolol as a single dose decreased by approximately 10% after coadministration with a single dose of moxifloxacin.
Calcium. 12 healthy volunteers were simultaneously treated with moxifloxacin (400 mg single dose) and calcium (500 mg single dose Ca++ supplement) followed by 2 additional doses of calcium 12 and 24 hours after moxifloxacin. Calcium had no significant effect on the AUC of moxifloxacin. Mean plasma Cmax was slightly reduced and plasma Tmax was increased when moxifloxacin was given with calcium compared to when moxifloxacin was given alone (2.5 hours vs. 0.9 hours). These differences are not considered clinically significant.
Digoxin. In a study of 12 healthy volunteers, there was no significant effect of moxifloxacin (400 mg once daily for 2 days) on the AUC of digoxin (0.6 mg as a single dose). The mean Cmax increased by approximately 50% during the distribution phase of digoxin. This transient increase in digoxin Cmax is not considered clinically significant. The pharmacokinetics of moxifloxacin were similar in the presence or absence of digoxin. When used concomitantly, no dose adjustment of moxifloxacin or digoxin is required.
Glibenclamide. In diabetic patients, the mean AUC and Cmax values of glibenclamide (2.5 mg once daily for 2 weeks before treatment and for 5 days when administered concomitantly) were 12 and 21% lower, respectively, when used with moxifloxacin (400 mg once daily for 5 days) compared with placebo. However, blood glucose levels were slightly reduced in patients taking glibenclamide and moxifloxacin compared with those taking glibenclamide alone, indicating that moxifloxacin did not influence the activity of glibenclamide. These interaction results are not considered clinically significant.
Itraconazole In a study of 11 healthy volunteers, there was no significant effect of itraconazole (200 mg once daily for 9 days), a strong CYP3A4 inhibitor, on the pharmacokinetics of moxifloxacin (single dose of 400 mg on day 7 of itraconazole). In addition, moxifloxacin has been shown to have no effect on the pharmacokinetics of itraconazole.
Morphine. In a study of 20 healthy male and female volunteers, there was no significant effect of morphine sulfate (10 mg IM single dose) on the mean AUC and Cmax of moxifloxacin (400 mg single dose).
Oral contraceptives. A placebo-controlled study in 29 healthy women showed that moxifloxacin, given at a dose of 400 mg/day for 7 days, did not affect the hormonal suppression caused by an oral contraceptive containing 0.15 mg levonorgestrel/0.03 mg ethinyl estradiol (as measured by serum progesterone, FSH, estradiol and LH), and on the pharmacokinetics of the active substances of the contraceptive taken.
Probenecid. In a study of 12 healthy volunteers, probenecid (500 mg twice daily for 2 days) did not affect the renal clearance or total amount of moxifloxacin (400 mg single dose) excreted through the kidneys.
Ranitidine. In a study of 10 healthy volunteers, there was no significant effect of ranitidine (150 mg 2 times daily for 3 days before moxifloxacin) on the pharmacokinetics of moxifloxacin (400 mg single dose).
Theophylline. In a study of 12 healthy volunteers, no significant effect of moxifloxacin (200 mg every 12 hours for 3 days) on the pharmacokinetics of theophylline (400 mg every 12 hours for 3 days) was found. In addition, theophylline has not been shown to affect the pharmacokinetics of moxifloxacin. The effect of simultaneous use of 400 mg of moxifloxacin 1 time per day with theophylline has not been studied.
Pharmaceutical interaction. Due to limited information regarding the compatibility of moxifloxacin in the form of a solution for intravenous administration with other drugs for intravenous administration, simultaneous infusion should not be carried out. Moxifloxacin solution is compatible with the following solutions in a ratio of 1:10 to 10:1: 0.9% sodium chloride, 1M sodium chloride, 5% dextrose, water for injection, 10% dextrose, lactated Ringer’s solution.
Overdose
Overdose
There are limited data on overdose with moxifloxacin. No side effects were observed when using moxifloxacin at a dose of up to 1200 mg once and 600 mg for 10 days or more. In case of overdose, one should focus on the clinical picture and carry out symptomatic maintenance therapy with ECG monitoring.
The use of activated charcoal immediately after oral administration of the drug may help prevent excessive systemic exposure to moxifloxacin in cases of overdose.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
Store in original packaging to protect from moisture.
Shelf life
Shelf life
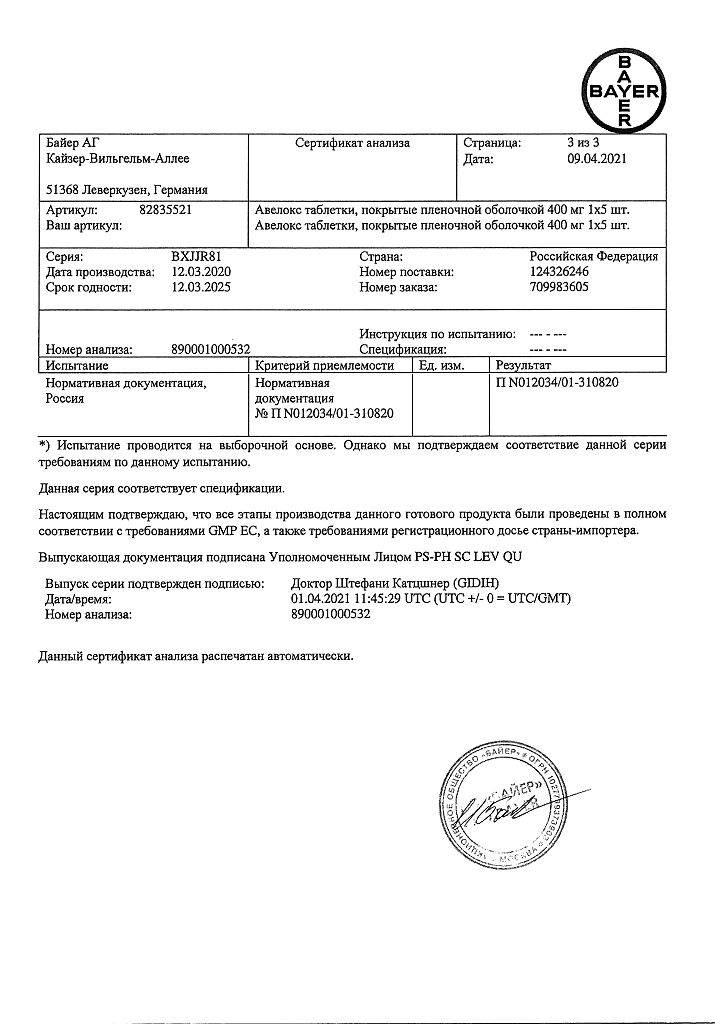
5 years. Do not use after expiration date.
Manufacturer
Manufacturer
Bayer Healthcare Manufacturing S.r.L., Italy
Additional information
| Shelf life | 5 years. Do not use after the expiration date. |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. Store in the original packaging to protect against moisture. |
| Manufacturer | Bayer Bitterfeld GmbH, Germany |
| Medication form | pills |
| Brand | Bayer Bitterfeld GmbH |
Related products
Buy Avelox, 400 mg, 5 pcs. with delivery to USA, UK, Europe and over 120 other countries.