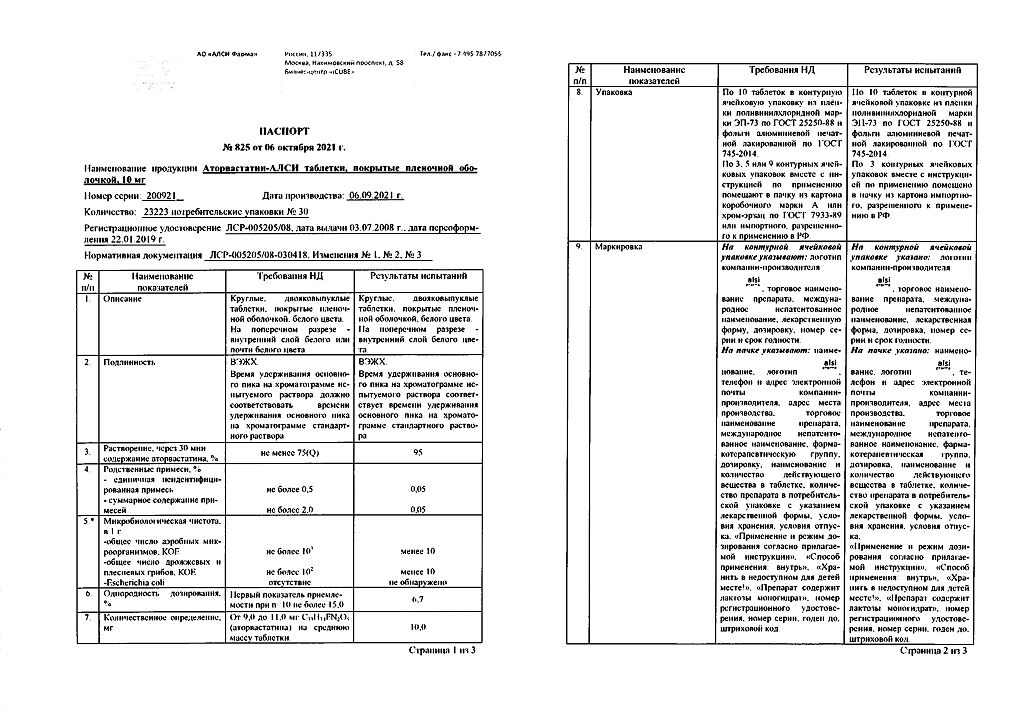
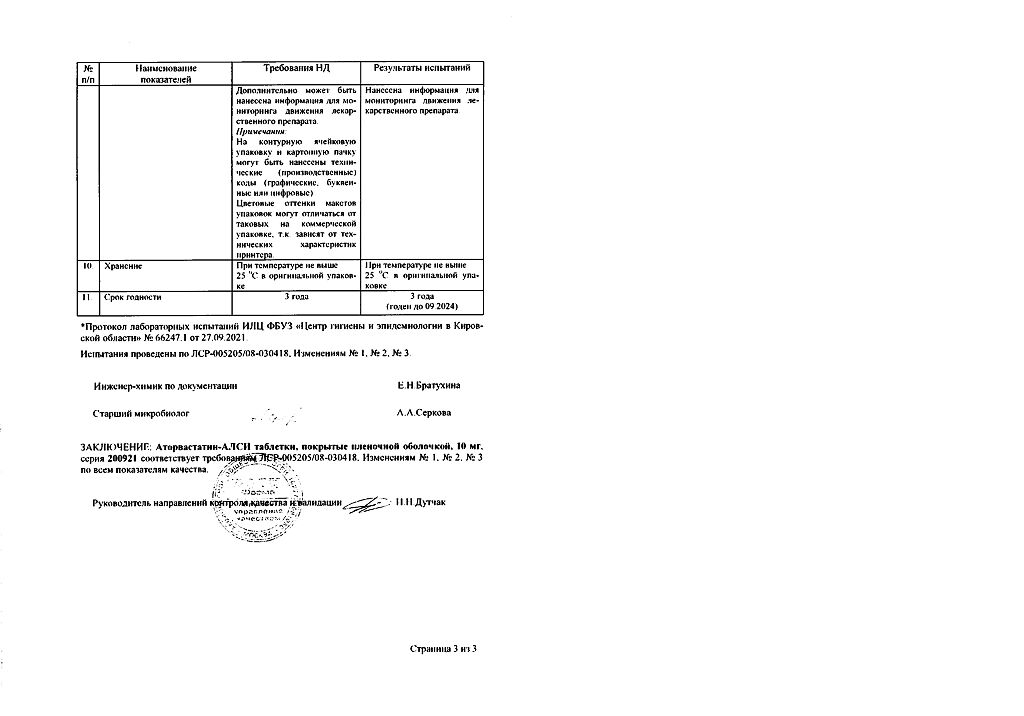
No products in the cart.
Atorvastatin-ALSI, 10 mg 30 pcs
€3.93 €3.49
Description
Reducing cholesterol, Preventing heart attacks and strokes
Atorvastatin is used:
- .in combination with diet to reduce elevated levels of total cholesterol, cholesterol/LDL, apolipoprotein B and triglycerides and increase HDL cholesterol in patients with primary hypercholesterolemia, heterozygous familial and non-familial hypercholesterolemia and combined (mixed) hyperlipidemia (Fredrickson types IIa and IIb);
- in combination with diet for treatment of patients with increased serum levels of triglycerides (Fredrickson type IV) and patients with dysbetalipoproteinemia (Fredrickson type III), in which diet therapy is not effective
- For lowering total cholesterol and LDL-cholesterol levels in patients with homozygous familial hypercholesterolemia when diet therapy and other non-pharmacological methods of treatment are not effective enough.
.
Indications
Indications
Atorvastatin is used:
in combination with diet to reduce elevated levels of total cholesterol, cholesterol/LDL, apolipoprotein B and triglycerides and increase HDL cholesterol in patients with primary hypercholesterolemia, heterozygous familial and non-familial hypercholesterolemia and combined (mixed) hyperlipidemia (Fredrickson types IIa and IIb);
in combination with diet for the treatment of patients with elevated serum triglyceride levels (Fredrickson type IV) and patients with dysbetalipoproteinemia (Fredrickson type III) in whom dietary therapy does not provide an adequate effect;
to reduce total cholesterol and LDL/LDL cholesterol levels in patients with homozygous familial hypercholesterolemia, when diet therapy and other non-pharmacological treatments are not effective enough.
Pharmacological effect
Pharmacological effect
A lipid-lowering drug from the group of statins.
Special instructions
Special instructions
Before starting Atorvastatin therapy, the patient must be prescribed a standard cholesterol-lowering diet, which he must follow during the entire treatment period.
The use of HMG-CoA reductase inhibitors to reduce blood lipid levels can lead to changes in biochemical parameters reflecting liver function. Liver function should be monitored before starting therapy, 6 weeks, 12 weeks after starting Atorvastatin and after each dose increase, and periodically, for example, every 6 months. An increase in the activity of liver enzymes in the blood serum may be observed during therapy with Atorvastatin. Patients who experience elevated enzyme levels should be monitored until enzyme levels return to normal. If alanine aminotransferase (ALT) or aspartic aminotransferase (AST) values are more than 3 times the upper acceptable limit, it is recommended to reduce the dose of Atorvastatin or discontinue treatment.
Atorvastatin should be used with caution in patients who abuse alcohol and/or have liver disease. Active liver disease or persistent increases in aminotransferase activity of unknown origin are contraindications to the use of Atorvastatin.
Treatment with Atorvastatin may cause myopathy. The diagnosis of myopathy (muscle pain and weakness in combination with an increase in creatine phosphokinase (CPK) activity more than 10 times the upper limit of normal) should be considered in patients with widespread myalgia, muscle soreness or weakness, and/or a marked increase in CPK activity. Patients should be warned that they should immediately tell their doctor if they experience unexplained muscle pain or weakness if they are accompanied by malaise or fever.
Atorvastatin therapy should be discontinued in the event of a marked increase in CPK activity or in the presence of confirmed or suspected myopathy. The risk of myopathy during treatment with other drugs in this class was increased with concomitant use of cyclosporine, fibrates, erythromycin, niacin, or azole antifungals. Many of these drugs inhibit cytochrome P450 3A4-mediated metabolism and/or drug transport. Atorvastatin is biotransformed by CYP 3A4.
When prescribing Atorvastatin in combination with fibrates, erythromycin, immunosuppressive agents, azole antifungals or nicotinic acid in lipid-lowering doses, the expected benefits and risks of treatment should be carefully weighed and patients should be regularly monitored for muscle pain or weakness, especially during the first months of treatment and during periods of increasing dosage of any drug. In such situations, periodic determination of CPK activity can be recommended, although such monitoring does not prevent the development of severe myopathy.
When using Atorvastatin, as well as other drugs of this class, cases of rhabdomyolysis with acute renal failure caused by myoglobinuria have been described. Atorvastatin therapy should be temporarily discontinued or completely discontinued if signs of possible myopathy appear or if there is a risk factor for the development of renal failure due to rhabdomyolysis (for example, severe acute infection, hypotension, major surgery, trauma, severe metabolic, endocrine and electrolyte disturbances, and uncontrolled seizures).
Before starting therapy with Atorvastatin, an attempt should be made to control hypercholesterolemia through adequate diet therapy, increased physical activity, weight loss in obese patients, and treatment of other conditions.
Patients should be warned to seek immediate medical attention if they experience unexplained muscle pain or weakness, especially if accompanied by malaise or fever.
Impact on the ability to drive a car and operate machinery
No adverse effects of Atorvastatin on the ability to drive or use machines have been reported.
Active ingredient
Active ingredient
Atorvastatin
Composition
Composition
One film-coated tablet contains:
active substance:
atorvastatin calcium trihydrate – 10.85 mg, which corresponds to 10 mg of atorvastatin;
excipients:
calcium carbonate – 33.00 mg,
microcrystalline cellulose – 48.00 mg,
lactose monohydrate (milk sugar) – 23.85 mg,
pregelatinized starch (starch 1500) – 32.80 mg,
colloidal silicon dioxide (Aerosil) – 0.75 mg,
magnesium stearate – 0.75 mg,
polyvinyl alcohol – 2.50 mg,
macrogol (polyethylene glycol) – 1.26 mg,
talc – 0.93 mg,
titanium dioxide – 1.56 mg.
Contraindications
Contraindications
hypersensitivity to the components of the drug;
active liver disease or increased activity of liver enzymes of unknown origin (more than 3 times the upper limit of normal);
liver failure (severity according to Child-Pugha classification A and B)
pregnancy;
lactation period;
age under 18 years (efficacy and safety have not been established).
With caution: alcohol abuse, history of liver disease, severe electrolyte imbalance, endocrine and metabolic disorders, arterial hypotension, severe acute infections (sepsis), uncontrolled epilepsy, major surgical interventions, trauma, skeletal muscle diseases.
Interaction
Interaction
The risk of myopathy during treatment with other drugs in this class is increased by concomitant use of cyclosporine, fibrates, erythromycin, azole antifungals, and nicotinic acid.
When simultaneous oral administration of Atorvastatin and a suspension containing magnesium and aluminum hydroxide, plasma concentrations of Atorvastatin decreased by approximately 35%, but the degree of reduction in LDL/cholesterol levels did not change.
With simultaneous use, Atorvastatin does not affect the pharmacokinetics of antipyrine (phenazone), so interaction with other drugs metabolized by the same cytochrome isoenzymes is not expected.
With simultaneous use of colestipol, plasma concentrations of Atorvastatin were reduced by approximately 25%. However, the lipid-lowering effect of the combination of Atorvastatin and colestipol was superior to that of each drug alone.
With repeated administration of digoxin and Atorvastatin at a dose of 10 mg, the equilibrium concentrations of digoxin in the blood plasma did not change. However, when using digoxin in combination with Atorvastatin at a dose of 80 mg/day. digoxin concentration increased by approximately 20%. Patients receiving digoxin in combination with Atorvastatin should be monitored.
With the simultaneous use of Atorvastatin and erythromycin (500 mg 4 times / day) or clarithromycin (500 mg 2 times / day), which inhibit cytochrome P450 3A4, an increase in the concentration of Atorvastatin in the blood plasma was observed.
With simultaneous use of Atorvastatin (10 mg 1 time / day) and azithromycin (500 mg 1 time / day), the concentration of Atorvastatin in the blood plasma did not change.
Atorvastatin did not have a clinically significant effect on the plasma concentration of terfenadine, which is metabolized mainly by cytochrome P450 3A4; in this regard, it seems unlikely that Atorvastatin can significantly affect the pharmacokinetic parameters of other cytochrome P450 3A4 substrates.
When atorvastatin was coadministered with an oral contraceptive containing norethindrone and ethinyl estradiol, significant increases in the AUC of norethindrone and ethinyl estradiol by approximately 30% and 20%, respectively, were observed. This effect should be taken into account when choosing an oral contraceptive for a woman receiving Atorvastatin.
Concomitant use with drugs that reduce the concentration of endogenous steroid hormones (including cimetidine, ketoconazole, spironolactone) increases the risk of a decrease in endogenous steroid hormones (caution should be exercised).
When studying the interaction of Atorvastatin with warfarin and cimetidine, no signs of clinically significant interaction were found.
With simultaneous use of Atorvastatin 80 mg and amlodipine 10 mg, the pharmacokinetics of Atorvastatin at steady state did not change.
There were no clinically significant adverse interactions between Atorvastatin and antihypertensive drugs.
The simultaneous use of Atorvastatin with protease inhibitors, known as cytochrome P450 3A4 inhibitors, was accompanied by an increase in the concentration of Atorvastatin in the blood plasma.
No pharmaceutical incompatibilities are known.
Overdose
Overdose
Treatment: there is no specific antidote; symptomatic therapy is carried out.
Hemodialysis is ineffective.
Recommendations for use
Recommendations for use
Before prescribing Atorvastatin, the patient should be recommended a standard lipid-lowering diet, which he should continue to follow throughout the entire period of therapy.
The initial dose is on average 10 mg 1 time / day. The dose varies from 10 to 80 mg 1 time/day.
The drug can be taken at any time of the day with food or regardless of meal time. The dose is adjusted based on baseline LDL/C cholesterol levels, goals of therapy, and individual response. At the beginning of treatment and/or during dose increases of Atorvastatin, it is necessary to monitor plasma lipid levels every 2-4 weeks and adjust the dose accordingly.
Primary hypercholesterolemia and mixed hyperlipidemia, as well as Fredrickson types III and IV.
In most cases, a dose of 10 mg of Atorvastatin once a day is sufficient. A significant therapeutic effect is observed after 2 weeks, as a rule, and the maximum therapeutic effect is usually observed after 4 weeks. With long-term treatment, this effect persists.
Homozygous familial hypercholesterolemia.
Prescribed at a dose of 80 mg (4 tablets of 20 mg) 1 time per day.
The use of the drug in patients with renal failure and kidney disease does not affect the level of Atorvastatin in the blood plasma or the degree of reduction in cholesterol/LDL when used, so no change in the dose of the drug is required.
In case of liver failure, the dose must be reduced (see section “With caution” and “Special instructions”).
When using the drug in elderly patients, there were no differences in safety, effectiveness, or achievement of lipid-lowering therapy goals compared to the general population.
Functional features
Functional features
Absorption is high. The maximum concentration (Cmax) in the blood plasma is achieved after 1-2 hours, Cmax in women is 20% higher, the area under the curve/concentration, time (AUC) is 10% lower; Cmax in patients with alcoholic cirrhosis is 16 times, AUC is 11 times higher than normal.
Food slightly reduces the rate and duration of drug absorption (by 25% and 9%, respectively), but the reduction in LDL cholesterol is similar to that when using Atorvastatin without food. The concentration of Atorvastatin when used in the evening is lower than in the morning (by approximately 30%). A linear relationship was revealed between the degree of absorption and the dose of the drug.
Bioavailability – 12%, systemic bioavailability of inhibitory activity against HMG-CoA reductase – 30%. Low systemic bioavailability is due to first-pass metabolism in the mucous membrane of the gastrointestinal tract and during the “first pass” through the liver.
The average volume of distribution is 381 l, the connection with blood plasma proteins is 98%. Metabolized primarily in the liver under the influence of cytochrome CYP3A4, CYP3A5 and CYP3A7 with the formation of pharmacologically active metabolites (ortho- and parahydroxylated derivatives, beta-oxidation products). In vitro, ortho- and parahydroxylated metabolites have an inhibitory effect on HMG-CoA reductase comparable to that of Atorvastatin. The inhibitory effect of the drug on HMG-CoA reductase is approximately 70% determined by the activity of circulating metabolites.
Excreted in bile after hepatic and/or extrahepatic metabolism (not subject to pronounced enterohepatic recirculation).
The half-life is 14 hours. Inhibitory activity against HMG-CoA reductase lasts for about 20-30 hours due to the presence of active metabolites. Less than 2% of the dose taken orally is determined in the urine. It is not excreted during hemodialysis.
Storage conditions
Storage conditions
In a dry place, protected from light, at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
ALSI Pharma, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | In a dry, light-protected place at a temperature not exceeding 25°C. Store out of the reach of children. |
| Manufacturer | ALSI Pharma, Russia |
| Medication form | pills |
| Brand | ALSI Pharma |
Other forms…
Related products
Buy Atorvastatin-ALSI, 10 mg 30 pcs with delivery to USA, UK, Europe and over 120 other countries.