No products in the cart.
Atoris, 40 mg 30 pcs.
€11.05 €9.67
Description
Prevention of heart attacks and strokes, Cholesterol, Reduction of cholesterol, Atherosclerosis
Hypercholesterolemia:
– to reduce elevated total cholesterol, LDL-C in adults with homozygous familial hypercholesterolemia as an adjunct to other hypolipidemic therapies (such as LDL-apheresis), or if such therapies are not available.
– Prevention of cardiovascular diseases:
– prevention of cardiovascular events in adult patients at high risk of developing primary cardiovascular events, as an adjunct to correction of other risk factors;
– secondary prevention of cardiovascular complications in patients with CHD to reduce mortality, MI, stroke, repeat hospitalizations for angina and the need for revascularization.
Indications
Indications
Hypercholesterolemia:
– as an adjunct to diet to reduce elevated total cholesterol, LDL-C, apo-B and TG in adults, adolescents and children 10 years of age or older with primary hypercholesterolemia, including familial hypercholesterolemia (heterozygous variant) or combined (mixed variant) hyperlipidemia (Fredrickson type IIa and IIb, respectively), when responsive to diet and other non-drug treatments insufficient;
– to reduce elevated total cholesterol and LDL cholesterol in adults with homozygous familial hypercholesterolemia as an adjunct to other lipid-lowering treatments (eg, LDL apheresis), or if such treatments are not available.
· Prevention of cardiovascular diseases:
– prevention of cardiovascular events in adult patients at high risk of developing primary cardiovascular events, as an addition to the correction of other risk factors;
– secondary prevention of cardiovascular complications in patients with coronary artery disease in order to reduce mortality, MI, strokes, re-hospitalization for angina pectoris and the need for revascularization.
Pharmacological effect
Pharmacological effect
lipid-lowering agent – HMG-CoA reductase inhibitor
Special instructions
Special instructions
In patients with risk factors for the development of rhabdomyolysis (impaired renal function, hypothyroidism, a patient’s history or family history of hereditary muscle disorders, previous toxic effects of HMG-CoA reductase inhibitors [statins] or fibrates on muscle tissue, a history of liver disease and/or patients who drink significant amounts of alcohol, age over 70 years, situations in which increased concentrations of atorvastatin are expected in patients blood plasma [for example, interactions with other drugs]).
Contraindicated in persons under 18 years of age (there is insufficient clinical data on the effectiveness and safety of the drug in this age group), with the exception of heterozygous familial hypercholesterolemia (use is contraindicated in children under 10 years of age).
Use in children from 10 to 18 years of age with heterozygous familial hypercholesterolemia.
The recommended starting dose is 10 mg 1 time per day. The dose may be increased to 80 mg per day depending on clinical effect and tolerability.
In the 3-year study, there were no clinically significant effects on growth and puberty as measured by the Global Maturation and Development Assessment, Tanner Stage Assessment, and Height and Weight Measurements.
In an 8-week open-label study, children with a Tanner score of 1 (N = 15) and ≥ 2 (N = 24) (aged 6–17 years) with heterozygous familial hypercholesterolemia and a baseline LDL-C concentration ≥ 4 mmol/L received atorvastatin therapy as 5 mg or 10 mg chewable tablets or film-coated tablets. shell, at a dose of 10 mg or 20 mg 1 time per day, respectively. The only significant covariate in the pharmacokinetic model of the atorvastatin population was body weight. The apparent clearance of atorvastatin in children did not differ from that in adult patients when allometrically measured by body weight. Over the range of action of atorvastatin and o-hydroxyatorvastatin, a consistent decrease in LDL-C and cholesterol was observed.
Liver dysfunction
If liver function is impaired, the dose of Atoris® should be reduced with regular monitoring of the serum activity of liver transaminases: aspartate aminotransferase (AST) and alanine aminotransferase (ALT).
Renal dysfunction
Impaired renal function does not affect the concentration of atorvastatin or the degree of reduction in plasma LDL-C concentrations, so no dose adjustment is required.
Elderly patients
No differences were found in the therapeutic efficacy and safety of Atoris® in elderly patients compared to the general population; no dose adjustment is required.
Effect on the liver
As with the use of other lipid-lowering drugs of this class, during treatment with atorvastatin, a moderate increase (more than 3 times compared with the upper limit of normal) in the activity of hepatic transaminases AST and ALT in the blood plasma was noted. A persistent increase in serum activity of hepatic transaminases (more than 3 times the upper limit of normal) was observed in 0.7% of patients receiving atorvastatin. The incidence of such changes when using atorvastatin at doses of 10 mg, 20 mg, 40 mg and 80 mg was 0.2%, 0.2%, 0.6% and 2.3%, respectively. An increase in the activity of “liver” transaminases in the blood plasma was usually not accompanied by jaundice or other clinical manifestations. When the dose of atorvastatin was reduced, or the drug was temporarily or completely discontinued, the activity of “liver” transaminases in the blood plasma returned to the initial level. Most patients continued to take atorvastatin at a reduced dose without any clinical consequences.
Before starting therapy, 6 weeks and 12 weeks after starting the use of Atoris®, or after increasing its dose, liver function tests should be monitored. Liver function should also be monitored when clinical signs of liver damage appear. In case of increased activity of “liver” transaminases in the blood plasma, the activity of ALT and AST in the blood plasma should be monitored until it returns to normal. If an increase in the activity of AST or ALT in the blood plasma by more than 3 times compared to the upper limit of normal persists, it is recommended to reduce the dose or discontinue the drug Atoris® (see section “Side effects”).
Atoris® should be used with caution in patients who drink significant amounts of alcohol and/or have a history of liver disease. Active liver disease or persistently increased activity of hepatic transaminases of unknown origin are a contraindication to the use of the drug Atoris® (see section “Contraindications”).
Action on skeletal muscles
Myalgia was observed in patients receiving atorvastatin (see section “Side effects”). The diagnosis of myopathy should be considered in patients with diffuse myalgia, muscle soreness or weakness, and/or a marked increase in serum CPK activity (more than 10 times the upper limit of normal). Therapy with Atoris® should be discontinued in the event of a marked increase in serum CPK activity, in the presence of confirmed myopathy or suspicion of its development. The risk of developing myopathy increases with the simultaneous use of drugs that increase the concentration of atorvastatin in the blood plasma (see sections “Interaction with other drugs” and “Pharmacological properties. Pharmacokinetics”), such as potent inhibitors of the CYP3A4 isoenzyme or carrier proteins (for example, cyclosporine, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole and HIV protease inhibitors, including ritonavir, lopinavir, atazanavir, indinavir, darunavir, tipranavir/ritonavir, etc.), gemfibrozil or other fibrates, antiviral drugs for the treatment of HCV (boceprevir, telaprevir, elbasvir/grazoprevir), erythromycin, nicotinic acid in lipid-lowering doses (more than 1 g/day), ezetimibe, azole antifungals, colchicine. Many of these drugs inhibit CYP3A4-mediated metabolism and/or drug transport. It is known that the CYP3A4 isoenzyme is the main liver isoenzyme involved in the biotransformation of atorvastatin. When using the drug Atoris® in combination with fibrates, erythromycin, immunosuppressants, azole antifungals or nicotinic acid in lipid-lowering doses (more than 1 g / day), the doctor must carefully weigh the expected benefits of treatment and the possible risks. Patients should be regularly monitored for muscle pain or weakness, especially during the first months of therapy and during dosage increases of any of these agents. If combination therapy is necessary, the use of lower initial and maintenance doses of the above drugs should be considered (see section “Dosage and Administration”). The simultaneous use of atorvastatin and fusidic acid is not recommended, therefore, temporary withdrawal of atorvastatin is recommended during treatment with fusidic acid. In such situations, periodic monitoring of serum CPK activity can be recommended, although such monitoring does not prevent the development of severe myopathy (see section “Interaction with other drugs”).
Before treatment
Atorvastatin should be prescribed with caution to patients with factors predisposing to the development of rhabdomyolysis. Before starting atorvastatin therapy, CPK activity in the blood plasma should be monitored in the following cases:
renal dysfunction,
· hypothyroidism,
· the patient has a history or family history of hereditary muscle disorders,
· already suffered toxic effects of HMG-CoA reductase inhibitors (statins) or fibrates on muscle tissue,
history of liver disease and/or patients drinking alcohol in significant quantities,
· in patients over the age of 70 years, the need to monitor CPK in the blood plasma should be assessed, given that these patients already have factors predisposing to the development of rhabdomyolysis,
· situations in which an increase in the concentration of atorvastatin in the blood plasma is expected, such as interactions with other drugs (see section “Interaction with other drugs”).
In such situations, the risk/benefit ratio should be assessed and medical monitoring of the patient’s condition should be carried out.
In case of a significant increase in serum CPK activity (more than 5 times the upper limit of normal), atorvastatin therapy should not be started.
When using the drug Atoris®, as well as other HMG-CoA reductase inhibitors, rare cases of rhabdomyolysis with acute renal failure caused by myoglobinuria have been described. A risk factor for the development of rhabdomyolysis may be pre-existing renal impairment. Such patients should be provided with more careful monitoring of the musculoskeletal system. If symptoms of myopathy appear or there are risk factors for the development of renal failure due to rhabdomyolysis (for example, severe acute infection, arterial hypotension, major surgery, trauma, metabolic, endocrine and fluid-electrolyte disturbances, uncontrolled convulsions), therapy with Atoris® should be temporarily discontinued or completely discontinued.
Very rare cases of the development of immune-mediated necrotizing myopathy have been reported during therapy or upon discontinuation of statin use. Immune-mediated necrotizing myopathy is clinically characterized by persistent proximal muscle weakness and increased serum CPK activity that persist despite discontinuation of statin treatment.
Attention! Patients should be warned to seek immediate medical attention if they experience unexplained pain or muscle weakness, especially if accompanied by malaise or fever.
Prevention of stroke by actively reducing plasma cholesterol concentrations (SPARCL)
In a retrospective analysis of stroke subtypes, a higher incidence of hemorrhagic stroke was observed in patients without coronary artery disease with a recent stroke or TIA initially receiving atorvastatin 80 mg/day compared with patients receiving placebo. The increased risk was particularly evident in patients with a history of hemorrhagic stroke or lacunar infarction at baseline. In this group of patients, the benefit/risk ratio when taking atorvastatin at a dose of 80 mg/day has not been determined; therefore, before starting therapy, the possible risk of hemorrhagic stroke in such patients should be carefully assessed.
After a special analysis of a clinical trial involving 4731 patients without coronary artery disease who had a stroke or TIA within the previous 6 months who were prescribed atorvastatin 80 mg/day, a higher incidence of hemorrhagic stroke was found in the atorvastatin group compared with the placebo group (55 in the atorvastatin group vs. 33 in the placebo group). Patients with hemorrhagic stroke at the time of inclusion in the study had a higher risk of recurrent hemorrhagic stroke (7 in the atorvastatin group versus 2 in the placebo group). However, patients receiving atorvastatin 80 mg/day had fewer strokes of any type (265 vs. 311) and fewer cardiovascular events (123 vs. 204).
Diabetes mellitus
Some evidence suggests that HMG-CoA reductase inhibitors (statins) as a class may lead to elevated blood glucose concentrations, and some patients at high risk for diabetes may develop a hyperglycemic state that requires treatment as in diabetes mellitus. However, this risk does not outweigh the benefits of therapy with HMG-CoA reductase inhibitors (statins) in terms of vascular risks, so this cannot be a reason to discontinue therapy. Patients at risk (fasting blood glucose concentration from 5.6 to 6.9 mmol/l, BMI > 30 kg/m2 body surface area, increased plasma TG concentration, arterial hypertension) should be under medical supervision, including monitoring of blood biochemical parameters, in accordance with national recommendations.
Interstitial lung disease
During therapy with certain HMG-CoA reductase inhibitors (statins), especially during long-term therapy, isolated cases of interstitial lung disease have been reported. Shortness of breath, nonproductive cough, and deterioration in general health (fatigue, weight loss, and fever) may occur. If interstitial lung disease is suspected in a patient, atorvastatin therapy should be discontinued.
Endocrine function
When using HMG-CoA reductase inhibitors (statins), including atorvastatin, cases of increased HbA1 concentration and fasting blood glucose concentration were observed. However, the risk of hyperglycemia is lower than the degree of reduction in the risk of vascular complications while taking HMG-CoA reductase inhibitors (statins).
Use in children
In the 3-year study, there were no clinically significant effects on growth and puberty as measured by the Global Maturation and Development Assessment, Tanner Stage Assessment, and Height and Weight Measurements.
Special information on excipients
The drug Atoris® contains lactose and is therefore contraindicated in the following conditions: lactose intolerance, lactase deficiency, glucose-galactose malabsorption syndrome.
There is no data on the effect of Atoris® on the ability to drive vehicles and engage in potentially hazardous activities that require increased concentration and speed of psychomotor reactions. However, given the possibility of dizziness, caution should be exercised when performing these activities.
Active ingredient
Active ingredient
Atorvastatin
Composition
Composition
1 film-coated tablet contains: Core
Active ingredient:
Atorvastatin calcium 41.44 mg, equivalent to atorvastatin 40.00 mg
Excipients:
Povidone-K25, sodium lauryl sulfate, calcium carbonate, microcrystalline cellulose, lactose monohydrate, croscarmellose sodium, crospovidone, magnesium stearate
Film casing
Opadry white Y-1-7000*
* Opadry white Y-1-7000:
Hypromellose, titanium dioxide (E171), macrogol-400
Pregnancy
Pregnancy
Atoris® is contraindicated during pregnancy.
Women of reproductive age should use adequate contraception during treatment. The use of Atoris® is contraindicated in women of childbearing age who do not use adequate methods of contraception.
Rare cases of congenital anomalies have been reported after fetal exposure to HMG-CoA reductase inhibitors (statins) in utero. Animal studies have shown toxic effects on reproductive function. Atoris® is contraindicated during breastfeeding. It is not known whether atorvastatin is excreted in breast milk. If it is necessary to prescribe the drug during lactation, breastfeeding must be stopped to avoid the risk of adverse events in infants.
Contraindications
Contraindications
– Hypersensitivity to any component of the drug.
– Active liver disease or an increase in the activity of “liver” transaminases in the blood plasma of unknown origin by more than 3 times compared with the upper limit of normal.
– Pregnancy.
– Breastfeeding period.
– Women of childbearing age who are not using adequate methods of contraception.
– Age up to 18 years (there is insufficient clinical data on the effectiveness and safety of the drug in this age group), with the exception of heterozygous familial hypercholesterolemia (use is contraindicated in children under 10 years of age).
– Simultaneous use with fusidic acid.
– Therapy with antiviral drugs for hepatitis C virus (HCV) glecaprevir/pibrentasvir.
– Lactase deficiency, lactose intolerance, glucose-galactose malabsorption syndrome, since Atoris® contains lactose.
Side Effects
Side Effects
Atoris® is usually well tolerated; adverse reactions are usually mild and transient.
Classification of the frequency of side effects recommended by the World Health Organization (WHO):
very common ≥ 1/10
often ≥ 1/100 to < 1/10
uncommon ≥ 1/1000 to < 1/100
rarely from ≥ 1/10000 to < 1/1000
very rare <1/10000
frequency unknown cannot be estimated from available data.
Infectious and parasitic diseases:
often: nasopharyngitis.
Blood and lymphatic system disorders:
rarely: thrombocytopenia.
Immune system disorders:
often: allergic reactions;
very rare: anaphylaxis.
Metabolic and nutritional disorders:
often: hyperglycemia;
uncommon: hypoglycemia, weight gain, anorexia;
frequency unknown: diabetes mellitus (incidence depends on the presence or absence of risk factors [fasting blood glucose concentration ≥ 5.6 mmol/l, body mass index [BMI] > 30 kg/m2 body surface area, elevated plasma TG concentrations, history of hypertension]).
Mental disorders:
infrequently: nightmares, insomnia;
frequency unknown: depression.
Nervous system disorders:
often: headache;
uncommon: dizziness, paresthesia, hypoesthesia, impaired taste perception, amnesia;
rarely: peripheral neuropathy;
frequency unknown: memory loss or decline.
Visual disorders:
uncommon: the appearance of a “veil” before the eyes;
rarely: visual impairment.
Hearing and labyrinth disorders:
uncommon: tinnitus;
very rare: hearing loss.
Disorders of the respiratory system, chest and mediastinal organs:
often: sore throat, nosebleeds;
frequency unknown: isolated cases of interstitial lung disease (usually with long-term use).
Digestive system disorders:
often: constipation, flatulence, dyspepsia, nausea, diarrhea;
uncommon: vomiting, abdominal pain, belching, pancreatitis, abdominal discomfort.
Disorders of the liver and biliary tract:
uncommon: hepatitis;
rarely: cholestasis.
Disorders of the skin and subcutaneous tissues:
uncommon: urticaria, skin itching, skin rash, alopecia;
rarely: angioedema, bullous rash, polymorphic exudative erythema (including Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell’s syndrome).
Musculoskeletal and connective tissue disorders:
often: myalgia, arthralgia, pain in the limbs, muscle cramps, swelling of the joints, back pain, musculoskeletal pain;
uncommon: neck pain, muscle weakness;
rarely: myopathy, myositis, rhabdomyolysis, tendinopathy (in some cases with tendon rupture), muscle rupture;
very rarely: lupus-like syndrome;
frequency unknown: immune-mediated necrotizing myopathy.
Renal and urinary tract disorders:
very rare: secondary renal failure.
Disorders of the genital organs and breast:
uncommon: impotence;
very rare: gynecomastia.
General disorders and disorders at the injection site:
uncommon: malaise, asthenic syndrome, chest pain, peripheral edema, fatigue, fever.
Laboratory and instrumental data:
often: abnormal results of liver tests (AST and ALT) in blood plasma, increased activity of serum creatine phosphokinase (CPK);
uncommon: leukocyturia;
frequency unknown: increased concentration of glycosylated hemoglobin (HbAl).
Interaction
Interaction
During treatment with HMG-CoA reductase inhibitors, with simultaneous use of cyclosporine, fibrates, nicotinic acid in lipid-lowering doses (more than 1 g / day) or inhibitors of the CYP3A4 isoenzyme (for example, erythromycin, clarithromycin, antifungal agents – azole derivatives), the risk of developing myopathy increases (see section “Special instructions”).
CYP3A4 isoenzyme inhibitors
Since atorvastatin is metabolized by the CYP3A4 isoenzyme, simultaneous use of atorvastatin with inhibitors of the CYP3A4 isoenzyme may lead to an increase in the concentration of atorvastatin in the blood plasma. The degree of interaction and potentiation effect is determined by the variability of the effect on the CYP3A4 isoenzyme.
It was found that potent inhibitors of the CYP3A4 isoenzyme lead to a significant increase in the concentration of atorvastatin in the blood plasma. Concomitant use of strong CYP3A4 inhibitors (such as cyclosporine, telithromycin, clarithromycin, delavirdine, stiripentol, ketoconazole, voriconazole, itraconazole, posaconazole, some antiviral drugs used to treat HCV (for example, elbasvir/grazoprevir) and HIV protease inhibitors, including ritonavir, should be avoided whenever possible. lopinavir, atazanavir, indinavir, darunavir, etc.). If concomitant use of these drugs is necessary, initiating therapy at the lowest dose should be considered and the possibility of reducing the maximum dose of atorvastatin should be evaluated.
Moderate inhibitors of the CYP3A4 isoenzyme (for example, erythromycin, diltiazem, verapamil and fluconazole) may lead to increased plasma concentrations of atorvastatin. With the simultaneous use of HMG-CoA reductase inhibitors (statins) and erythromycin, an increased risk of developing myopathy was noted. Interaction studies between amiodarone or verapamil and atorvastatin have not been conducted. Both amiodarone and verapamil are known to inhibit CYP3A4 activity, and concomitant use of these drugs with atorvastatin may result in increased atorvastatin exposure. In this regard, it is recommended to reduce the maximum dose of atorvastatin and appropriately monitor the patient’s condition when used simultaneously with moderate inhibitors of the CYP3A4 isoenzyme. Monitoring should be carried out after the start of therapy and against the background of changing the dose of the CYP3A4 isoenzyme inhibitor.
Gemfibrozil/fibrates
With the use of fibrates in monotherapy, adverse reactions affecting the musculoskeletal system, including rhabdomyolysis, were periodically noted. The risk of such reactions increases with simultaneous use of fibrates and atorvastatin. If concomitant use of these drugs cannot be avoided, the minimum effective dose of atorvastatin should be used and patients should be monitored regularly.
Ezetimibe
The use of ezetimibe is associated with the development of adverse reactions from the musculoskeletal system, including the development of rhabdomyolysis. The risk of such reactions increases with simultaneous use of ezetimibe and atorvastatin. For such patients, careful monitoring is recommended.
Erythromycin/clarithromycin
With the simultaneous use of atorvastatin and erythromycin (500 mg 4 times a day) or clarithromycin (500 mg 2 times a day), inhibitors of the CYP3A4 isoenzyme, an increase in the concentration of atorvastatin in the blood plasma was observed (see sections “Pharmacological properties. Pharmacokinetics” and “Special instructions”).
Protease inhibitors
The simultaneous use of atorvastatin with protease inhibitors, known as inhibitors of the CYP3A4 isoenzyme, is accompanied by an increase in the concentration of atorvastatin in the blood plasma.
Diltiazem
The simultaneous use of atorvastatin at a dose of 40 mg with diltiazem at a dose of 240 mg leads to an increase in the concentration of atorvastatin in the blood plasma (see section “Pharmacological properties. Pharmacokinetics”).
Cimetidine
No clinically significant interaction of atorvastatin with cimetidine was detected (see section “Pharmacological properties. Pharmacokinetics”).
Itraconazole
The simultaneous use of atorvastatin in doses from 20 mg to 40 mg and itraconazole in a dose of 200 mg led to an increase in the AUC value of atorvastatin (see section “Pharmacological properties. Pharmacokinetics”).
Grapefruit juice
Since grapefruit juice contains one or more components that inhibit the CYP3A4 isoenzyme, its excessive consumption (more than 1.2 liters per day) may cause an increase in the concentration of atorvastatin in the blood plasma (see section “Pharmacological properties. Pharmacokinetics”).
Transport protein inhibitors
Atorvastatin is a substrate of the liver enzyme transporters, OATP1B1 and OATP1B3 transporters. Atorvastatin metabolites are substrates of OATP1B1. Atorvastatin is also identified as a substrate of the efflux transporters MDR1 and BCRP, which may limit the intestinal absorption and biliary clearance of atorvastatin (see section “Pharmacological properties. Pharmacokinetics”).
The simultaneous use of atorvastatin at a dose of 10 mg and cyclosporine at a dose of 5.2 mg/kg/day led to an increase in the level of systemic exposure to atorvastatin (increase in AUC by 8.7 times) (see section “Pharmacological properties. Pharmacokinetics”). Cyclosporine is an inhibitor of organic anion transport polypeptide 1B1 (OATP1B1), 1B3 (OATP1B3), protein associated with MDR1 and BCRP, as well as the isoenzyme CYP3A4, therefore, it increases the level of systemic exposure to atorvastatin. The daily dose of atorvastatin should not exceed 10 mg (see section “Dosage and Administration”).
Elbasvir and grazoprevir are inhibitors of OATP1B1, OATP1B3, MDR1 and BCRP, therefore, they increase the level of systemic exposure of atorvastatin. Atoris® should be used with caution and at the lowest dose required (see section “Method of administration and dosage”).
Inducers of the CYP3A4 isoenzyme
Concomitant use of atorvastatin with inducers of the CYP3A4 isoenzyme (for example, efavirenz, rifampicin or St. John’s wort preparations) may lead to a decrease in the concentration of atorvastatin in the blood plasma. Due to the dual mechanism of interaction with rifampicin (an inducer of the CYP3A4 isoenzyme and an inhibitor of the hepatocyte transport protein OATP1B1), simultaneous use of atorvastatin and rifampicin is recommended, since delayed administration of atorvastatin after taking rifampicin leads to a significant decrease in the concentration of atorvastatin in the blood plasma (see section “Pharmacological properties. Pharmacokinetics”). However, the effect of rifampicin on hepatocyte concentrations of atorvastatin is unknown, and if concomitant use cannot be avoided, the effectiveness of the combination should be carefully monitored during therapy.
Antacids
Simultaneous oral administration of a suspension containing magnesium hydroxide and aluminum hydroxide reduced the concentration of atorvastatin in the blood plasma (change in AUC: 0.66), but the degree of reduction in the concentration of LDL-C in the blood plasma did not change.
Phenazone
Atorvastatin does not affect the pharmacokinetics of phenazone, so interaction with other drugs metabolized by the same isoenzymes of the cytochrome system is not expected.
Colestipol
With simultaneous use of colestipol, the concentration of atorvastatin in the blood plasma decreased (change in AUC: 0.74), however, the lipid-lowering effect of the combination of atorvastatin and colestipol was superior to that of each drug separately.
Digoxin
With repeated administration of digoxin and atorvastatin at a dose of 10 mg, the equilibrium concentrations of digoxin in the blood plasma did not change. However, when digoxin was used in combination with atorvastatin at a dose of 80 mg/day, digoxin concentrations increased (AUC change: 0.74). Patients receiving digoxin concomitantly with atorvastatin require appropriate monitoring.
Azithromycin
With simultaneous use of atorvastatin at a dose of 10 mg 1 time per day and azithromycin at a dose of 500 mg 1 time per day, the concentration of atorvastatin in the blood plasma did not change.
Oral contraceptives
When atorvastatin was co-administered with oral contraceptives containing norethisterone and ethinyl estradiol, a significant increase in the AUC of norethisterone (AUC change: 1.28) and ethinyl estradiol (AUC change: 1.19) was observed. This effect should be taken into account when choosing an oral contraceptive drug for a woman taking atorvastatin.
Terfenadine
With simultaneous use of atorvastatin and terfenadine, no clinically significant changes in the pharmacokinetics of terfenadine were detected.
Warfarin
In a clinical study in patients regularly receiving warfarin therapy, concomitant use of atorvastatin 80 mg daily resulted in a small increase in prothrombin time of approximately 1.7 seconds during the first 4 days of therapy. The indicator returned to normal within 15 days of atorvastatin therapy. Although significant interactions affecting anticoagulant function have only been observed in rare cases, prothrombin time should be determined prior to initiation of atorvastatin therapy in patients receiving coumarin anticoagulant therapy and regularly during therapy to prevent significant changes in prothrombin time. Once stable prothrombin time values are observed, its monitoring can be carried out in the same way as recommended for patients receiving coumarin anticoagulants. When changing the dose of atorvastatin or discontinuing therapy, prothrombin time should be monitored according to the same principles as described above. Atorvastatin therapy was not associated with bleeding or changes in prothrombin time in patients not receiving anticoagulant treatment.
Colchicine
Although studies have not been conducted on the simultaneous use of colchicine and atorvastatin, there are reports of the development of myopathy when using this combination. Caution should be exercised when atorvastatin and colchicine are used concomitantly.
Amlodipine
In a drug interaction study in healthy volunteers, coadministration of atorvastatin 80 mg and amlodipine 10 mg resulted in a clinically insignificant increase in atorvastatin plasma concentrations (AUC change: 1.18).
Fusidic acid
During post-marketing studies, cases of rhabdomyolysis have been reported in patients taking concomitant statins, including atorvastatin and fusidic acid. The mechanism of this interaction is unknown. In patients for whom the use of fusidic acid is considered necessary, treatment with statins should be discontinued for the entire period of use of fusidic acid. Statin therapy can be resumed 7 days after the last dose of fusidic acid. In exceptional cases where long-term systemic therapy with fusidic acid is necessary, for example, for the treatment of severe infections, the need for simultaneous use of atorvastatin and fusidic acid should be considered on a case-by-case basis and under close medical supervision. The patient should seek immediate medical attention if symptoms of muscle weakness, tenderness, or pain occur.
Other concomitant therapy
In clinical studies, atorvastatin was used concomitantly with antihypertensive agents and estrogens as part of hormone replacement therapy. There were no signs of clinically significant adverse interactions, and no interaction studies with specific drugs were conducted.
In addition, an increase in the concentration of atorvastatin was observed when used simultaneously with HIV protease inhibitors (with combinations of lopinavir and ritonavir, saquinavir and ritonavir, darunavir and ritonavir, with fosamprenavir, with combinations of fosamprenavir and ritonavir and with nelfinavir), hepatitis C virus protease inhibitors (boceprevir, elbasvir/grazoprevir, simeprevir), clarithromycin and itraconazole. Caution should be exercised when using these drugs together and the lowest effective dose of atorvastatin should be used.
Overdose
Overdose
There is no specific antidote for the treatment of overdose with Atoris®. In case of overdose, symptomatic treatment should be provided as needed. Liver function tests should be performed and serum CPK activity should be monitored. Since atorvastatin actively binds to plasma proteins, hemodialysis is ineffective.
Storage conditions
Storage conditions
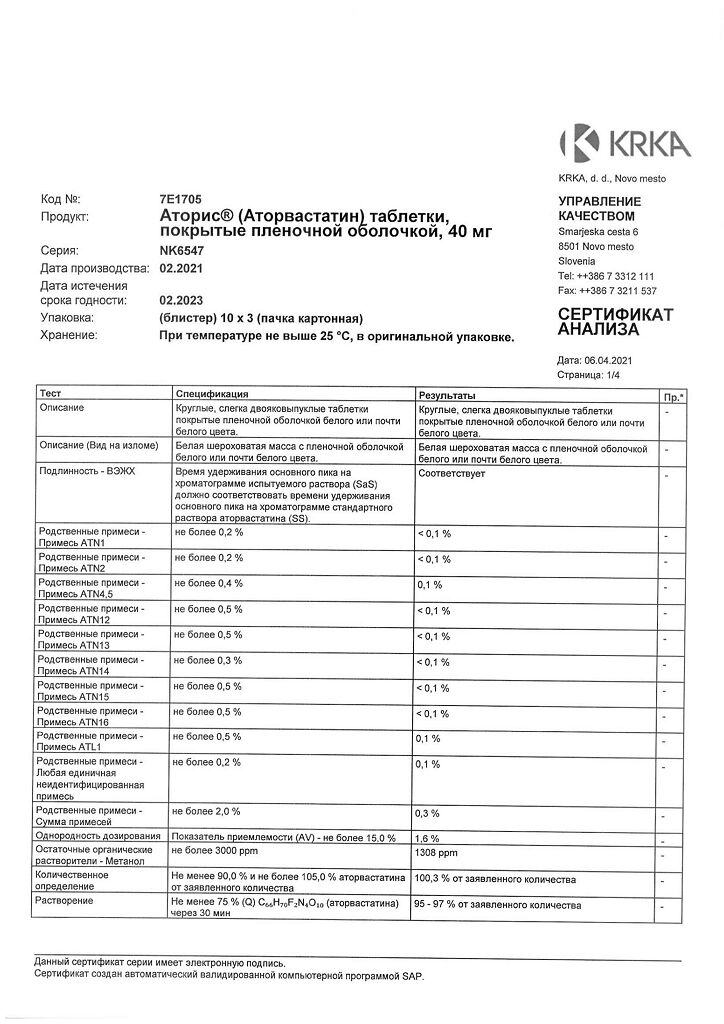
At a temperature not exceeding 25 °C, in the original packaging.
Keep out of the reach of children.
Shelf life
Shelf life
2 years.
Do not use the drug after the expiration date.
Manufacturer
Manufacturer
KRKA dd Novo Mesto, Slovenia
Additional information
| Shelf life | 2 years. Do not use the drug after the expiration date. |
|---|---|
| Conditions of storage | At the temperature not more than 25 ° C, in the original package. Keep out of reach of children. |
| Manufacturer | KRKA dd Novo mesto, Slovenia |
| Medication form | pills |
| Brand | KRKA dd Novo mesto |
Other forms…
Related products
Buy Atoris, 40 mg 30 pcs. with delivery to USA, UK, Europe and over 120 other countries.