No products in the cart.
Artrofoon, tablets 100 pcs
€12.39 €10.33
Description
Pharmacological properties
Pharmacodynamics
It was experimentally and clinically proved that the preparation modulates the production and functional activity of endogenous tumor necrosis factor alpha (TNFα) in rheumatoid arthritis, osteoarthritis, osteochondrosis, back pain; nonspecific ulcerative colitis (NUC). It has anti-inflammatory and analgesic effect.
By reducing the number of pro-inflammatory cytokines and inflammatory mediators it prevents the progression of inflammatory damages of tissues and target organs in inflammatory and degenerative joint diseases and IPP.
Pharmacokinetics
The sensitivity of modern physico-chemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, chromato-mass spectrometry) does not allow evaluating the ultra-low doses of antibodies in biological fluids, organs and tissues, which makes it technically impossible to study the pharmacokinetics of the drug Artrofoon.
Indications
Indications
The drug Artrofoon® is indicated for use in adults.
Rheumatoid arthritis, osteoarthritis (including spondyloarthritis) and other joint diseases. During an exacerbation, it is used as part of complex therapy (with non-steroidal anti-inflammatory drugs). During the period of remission, the drug can be used as monotherapy. Long-term (course) use of the drug contributes to a longer period of remission.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group
Anti-inflammatory agent.
ATX code
M01.
Pharmacological properties
Pharmacodynamics
It has been experimentally and clinically proven that the drug modulates the production and functional activity of endogenous tumor necrosis factor alpha (TNFα) in rheumatoid arthritis, osteoarthritis, osteochondrosis, and back pain. Has anti-inflammatory and analgesic effects. By reducing the production of a number of pro-inflammatory cytokines and inflammatory mediators, it prevents the progression of inflammatory damage to tissues and target organs in inflammatory-degenerative joint diseases.
Pharmacokinetics
The sensitivity of modern physicochemical methods of analysis (gas-liquid chromatography, high-performance liquid chromatography, gas chromatography-mass spectrometry) does not allow assessing the content of the active substance in biological fluids, organs and tissues, which makes it technically impossible to study the pharmacokinetics of the drug Artrofoon®.
Special instructions
Special instructions
On days 3-5 after the start of treatment, a moderately expressed transient increase in pain or local manifestations of inflammation is possible, which does not require changes in the pharmacotherapy regimen. In some cases, with a pronounced increase in pain or local signs of inflammation, it is necessary to temporarily reduce the dose to 1-2 tablets per day.
If you have an intolerance to certain sugars, consult your doctor before taking this drug.
Impact on the ability to drive vehicles and machinery
The drug Artrofoon® does not affect the ability to drive vehicles and other potentially dangerous mechanisms.
Active ingredient
Active ingredient
Antibodies
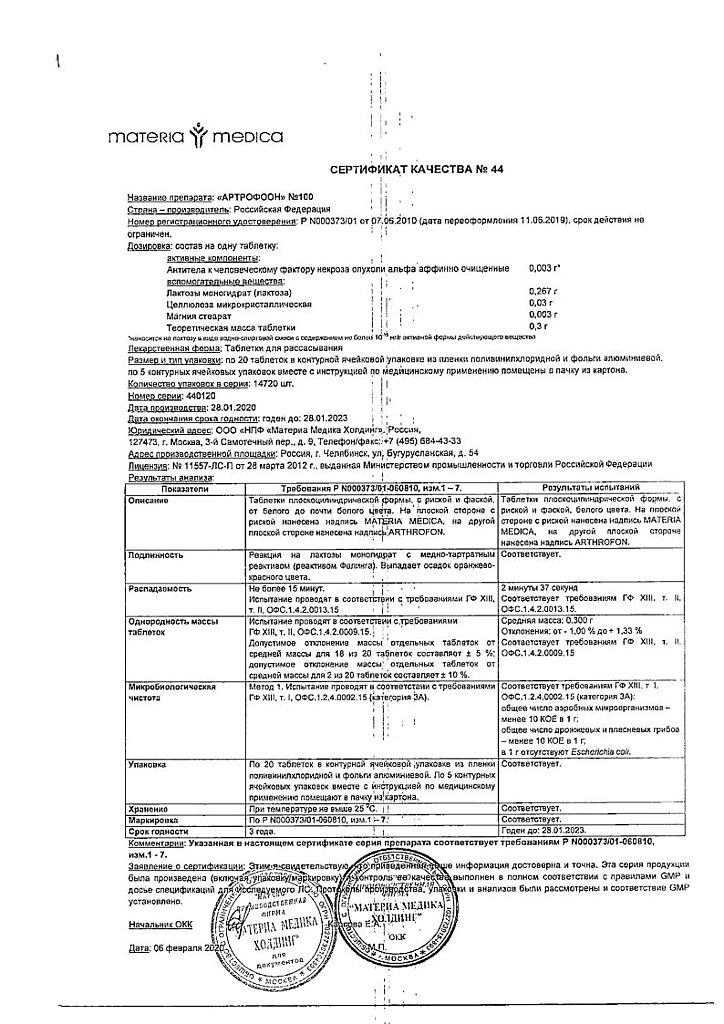
Composition
Composition
Active ingredient:
antibodies to human tumor necrosis factor alpha affinity
purified – 10,000 EMD*.
Excipients: lactose monohydrate, microcrystalline cellulose, magnesium stearate.
* EMD – units of modifying action.
Pregnancy
Pregnancy
The safety of using Artrofoon® in pregnant women and during lactation has not been studied. If it is necessary to take the drug, the benefit-risk ratio should be taken into account.
Contraindications
Contraindications
Increased individual sensitivity to the components of the drug.
Not recommended for use in children and adolescents under 18 years of age (due to lack of experience in clinical use).
Lactase deficiency, hereditary galactose intolerance, glucose-galactose malabsorption.
Side Effects
Side Effects
Reactions of increased individual sensitivity to the components of the drug are possible.
Interaction
Interaction
Cases of incompatibility with other drugs have not been reported to date. It is possible to combine the drug with non-steroidal anti-inflammatory drugs.
Overdose
Overdose
In case of overdose, dyspepsia may occur due to the excipients included in the drug.
Storage conditions
Storage conditions
At a temperature not exceeding 25 °C.
Keep out of the reach of children.
During the period of use of the drug, store the blister pack in a cardboard box provided by the manufacturer.
Shelf life
Shelf life
3 years.
Do not use after expiration date.
Manufacturer
Manufacturer
Materia Medica Holding, Russia
Additional information
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 ° C. Keep out of reach of children. |
| Manufacturer | Materiala Medica Holding, Russia |
| Medication form | lozenges |
| Brand | Materiala Medica Holding |
Related products
Buy Artrofoon, tablets 100 pcs with delivery to USA, UK, Europe and over 120 other countries.