No products in the cart.
AnviMax, 5 g cranberry 6 pcs
€7.71 €6.75
Description
Pharmacotherapeutic group: Acute respiratory infections and “colds” symptoms remedy.
ATX code: R05X
Pharmacological properties
Pharmacodynamics
Combination drug, has antiviral, interferonogenic, antipyretic, analgesic, antihistamine and angioprotective action.
Paracetamol has analgesic and antipyretic effects.
Ascorbic acid is involved in the regulation of redox processes, contributes to normal capillary permeability, blood coagulation, tissue regeneration, plays a positive role in the development of immune reactions in the body, makes up for the lack of vitamin C.
Calcium gluconate as a source of calcium ions prevents development of increased permeability and fragility of blood vessels and causes hemorrhagic processes during influenza and acute respiratory viral infection (ARI) and has anti-allergic effect (mechanism is unknown).
Rimantadine has antiviral activity against influenza A virus. By blocking the M2 channels of influenza A virus, it disrupts its ability to enter cells and release ribonucleoprotein, thereby inhibiting a crucial stage of viral replication. It induces production of interferon alpha and gamma. In influenza caused by B virus rimantadine has antitoxic effect.
Rutoside is an angioprotector. It reduces capillary permeability, swelling and inflammation, strengthens the vascular wall. Inhibits aggregation and increases the degree of deformation of red blood cells.
Loratadine is a blocker of H1-histamine receptors, prevents the development of tissue edema associated with the release of histamine.
Pharmacokinetics
Paracetamol. Absorption is high. Binding with plasma proteins – 15%. It penetrates through the blood-brain barrier. Metabolized in the liver in three main ways: conjugation with glucuronides, conjugation with sulfates, oxidation by liver microsomal enzymes. In the latter case toxic intermediate metabolites are formed, which subsequently conjugate with glutathione and then with cysteine and mercapturic acid. The main cytochrome P450 isoenzymes for this metabolic pathway are CYP2E1 (predominantly), CYP1A2 and CYP3A4 (secondary role). In glutathione deficiency, these metabolites can cause damage and necrosis of hepatocytes. Additional metabolic pathways are hydroxylation to 3-hydroxyparacetamol and methoxylation to 3-methoxy-paracetamol, which are subsequently conjugated to glucuronides or sulfates. In adults, glucuronidation predominates. Conjugated metabolites of paracetamol (glucuronides, sulfates and conjugates with glutathione) have low pharmacological (including toxic) activity. Excreted by the kidneys as metabolites, mainly conjugates, only 3% unchanged. In elderly patients, the drug clearance decreases and T1/2 increases.
The maximum concentration (Cmax) of paracetamol in plasma is reached after 0.7±0.39 hours when using the powder and is 4.79±1.81 µg/ml, the half-life (T1/2) is 2.73±0.76 hours.
Ascorbic acid is absorbed in the gastrointestinal tract (mainly in the jejunum). The binding to plasma proteins is 25%. Diseases of the gastrointestinal tract (gastric and 12 duodenal ulcers, constipation or diarrhea, worm infestation, giardiasis), consumption of fresh fruit and vegetable juices, alkaline drinking reduce absorption of ascorbic acid in the intestine. Normal plasma concentration of ascorbic acid is approximately 10-20 µg/ml. Time of maximum plasma concentration after oral administration is 4 hours. It penetrates easily into leukocytes, platelets and then into all tissues; the highest concentration is reached in glandular organs, leukocytes, liver and eye lens; it penetrates through the placenta. The concentration of ascorbic acid in leukocytes and platelets is higher than in erythrocytes and plasma. In deficiency states the concentration in leukocytes decreases later and more slowly and is considered a better criterion for assessing deficiency than the concentration in plasma. It is metabolized mainly in the liver to deoxyascorbic acid and further to oxalic acetic acid and ascorbate-2-sulfate. It is excreted by the kidneys, through the intestines, with sweat unchanged and as metabolites. Smoking and use of ethanol accelerate the breakdown of ascorbic acid (conversion to inactive metabolites), dramatically reducing the body’s reserves. It is excreted by hemodialysis.
Calcium gluconate. Approximately 1/5-1/3 of orally administered calcium gluconate is absorbed in the small intestine; this process depends on the presence of ergocalciferol, pH, dietary characteristics, and the presence of factors that can bind calcium ions. Absorption of calcium ions increases with calcium deficiency and with a diet low in calcium ions. About 20% is excreted by the kidneys, the rest (80%) by the intestines.
Rimantadine. After oral administration it is almost completely absorbed in the intestine. Absorption is slow. Binding to plasma proteins is about 40%. Volume of distribution – 17-25 l/kg. Concentration in nasal secretion is 50% higher than in plasma. Metabolized in the liver. More than 90% is excreted by the kidneys within 72 hours, mainly as metabolites, 15% unchanged. In chronic renal insufficiency the half-life is increased by 2 times. In patients with renal insufficiency and in the elderly the drug may accumulate in toxic concentrations if the dose is not adjusted in proportion to the decrease in creatinine clearance. Hemodialysis has little effect on rimantadine clearance.
The Cmax of rimantadine in plasma is reached with powder administration after 5.28±2.54 hours and is 69.0±19.7 ng/mL, T1/2 is 33.26±12.76 hours.
Rutoside. Time of maximum concentration in blood plasma after oral administration – 1-9 hours. It is excreted mainly with the bile and to a lesser extent by the kidneys. T1/2 is 10-25 h.
Loratadine. Rapidly and completely absorbed in the gastrointestinal tract. The maximum concentration in the elderly is increased by 50%. Binding with plasma proteins is 97%. It is metabolized in the liver with the formation of the active metabolite descarboethoxyloratadine with the participation of cytochrome isoenzymes CYP3A4 and to a lesser extent CYP2D6. It does not penetrate the blood-brain barrier. It is excreted by the kidneys and in bile. Pharmacokinetics is practically unchanged in patients with chronic renal insufficiency and in hemodialysis.
The Cmax of loratadine in plasma is reached after 3.28±1.25 hours and is 1.85±0.95 ng/mL, T1/2 is 11.29±5.52 hours.
Indications
Indications
Treatment of influenza type A, symptomatic treatment of “colds”, influenza and ARVI, accompanied by fever, chills, nasal congestion, sore throat, pain in joints and muscles, headache.
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: acute respiratory infections and “cold” symptoms – remedy.
ATX code: R05X
Pharmacological properties
Pharmacodynamics
The combined drug has antiviral, interferonogenic, antipyretic, analgesic, antihistamine and angioprotective effects.
Paracetamol has analgesic and antipyretic effects.
Ascorbic acid is involved in the regulation of redox processes, promotes normal capillary permeability, blood clotting, tissue regeneration, plays a positive role in the development of the body’s immune reactions, and replenishes vitamin C deficiency.
Calcium gluconate, as a source of calcium ions, prevents the development of increased permeability and fragility of blood vessels, causing hemorrhagic processes in influenza and acute respiratory viral infection (ARVI), and has an antiallergic effect (the mechanism is unclear).
Rimantadine has antiviral activity against the influenza A virus. By blocking the M2 channels of the influenza A virus, it disrupts its ability to penetrate cells and release ribonucleoprotein, thereby inhibiting the most important stage of viral replication. Induces the production of alpha and gamma interferons. For influenza caused by virus B, rimantadine has an antitoxic effect.
Rutoside is an angioprotector. Reduces capillary permeability, swelling and inflammation, strengthens the vascular wall. Inhibits aggregation and increases the degree of deformation of red blood cells.
Loratadine is a H1-histamine receptor blocker that prevents the development of tissue edema associated with the release of histamine.
Pharmacokinetics
Paracetamol. Absorption is high. Communication with plasma proteins – 15%. Penetrates the blood-brain barrier. Metabolized in the liver in three main ways: conjugation with glucuronides, conjugation with sulfates, oxidation by microsomal liver enzymes. In the latter case, toxic intermediate metabolites are formed, which are subsequently conjugated with glutathione, and then with cysteine and mercapturic acid. The main cytochrome P450 isoenzymes for this metabolic pathway are the CYP2E1 isoenzyme (mainly), CYP1A2 and CYP3A4 (minor role). With glutathione deficiency, these metabolites can cause damage and necrosis of hepatocytes. Additional metabolic pathways include hydroxylation to 3-hydroxyparacetamol and methoxylation to 3-methoxy-paracetamol, which are subsequently conjugated to glucuronides or sulfates. In adults, glucuronidation predominates. Conjugated metabolites of paracetamol (glucuronides, sulfates and conjugates with glutathione) have low pharmacological (including toxic) activity. Excreted by the kidneys in the form of metabolites, mainly conjugates, only 3% unchanged. In elderly patients, drug clearance decreases and T1/2 increases.
The maximum concentration (Cmax) of paracetamol in the blood plasma is achieved when using the powder after 0.7±0.39 hours and is 4.79±1.81 mcg/ml, the half-life (T1/2) is 2.73±0.76 hours.
Ascorbic acid is absorbed in the gastrointestinal tract (mainly in the jejunum). Communication with plasma proteins – 25%. Diseases of the gastrointestinal tract (peptic ulcer of the stomach and duodenum, constipation or diarrhea, helminthic infestation, giardiasis), consumption of fresh fruit and vegetable juices, alkaline drinking reduce the absorption of ascorbic acid in the intestine. The normal concentration of ascorbic acid in plasma is approximately 10-20 μg/ml. The time of maximum concentration in blood plasma after oral administration is 4 hours. Easily penetrates into leukocytes, platelets, and then into all tissues; the highest concentration is achieved in the glandular organs, leukocytes, liver and lens of the eye; penetrates the placenta. The concentration of ascorbic acid in leukocytes and platelets is higher than in erythrocytes and plasma. In deficiency states, leukocyte concentrations decline later and more slowly and are considered a better measure of deficiency than plasma concentrations. Metabolized primarily in the liver into deoxyascorbic acid and further into oxaloacetic acid and ascorbate-2-sulfate. It is excreted by the kidneys, through the intestines, with sweat, unchanged and in the form of metabolites. Smoking and drinking ethanol accelerate the destruction of ascorbic acid (conversion into inactive metabolites), sharply reducing reserves in the body. Excreted during hemodialysis.
Calcium gluconate. Approximately 1/5-1/3 of orally administered calcium gluconate is absorbed in the small intestine; this process depends on the presence of ergocalciferol, pH, diet and the presence of factors capable of binding calcium ions. The absorption of calcium ions increases with calcium deficiency and the use of a diet with a reduced content of calcium ions. About 20% is excreted by the kidneys, the rest (80%) by the intestines.
Rimantadine. After oral administration, it is almost completely absorbed from the intestines. Absorption is slow. Communication with plasma proteins is about 40%. Volume of distribution – 17-25 l/kg. The concentration in nasal secretions is 50% higher than the plasma concentration. Metabolized in the liver. More than 90% is excreted by the kidneys within 72 hours, mainly in the form of metabolites, 15% unchanged. In chronic renal failure, the half-life increases by 2 times. May accumulate in toxic concentrations in persons with renal impairment and in the elderly unless the dose is adjusted proportionately to the decrease in creatinine clearance. Hemodialysis has little effect on the clearance of rimantadine.
Cmax of rimantadine in blood plasma is achieved when using the powder after 5.28±2.54 hours and is 69.0±19.7 ng/ml, T1/2 – 33.26±12.76 hours.
Rutoside. The time of maximum concentration in blood plasma after oral administration is 1-9 hours. It is excreted mainly with bile and to a lesser extent by the kidneys. T1/2 – 10-25 hours.
Loratadine. Quickly and completely absorbed from the gastrointestinal tract. The maximum concentration in older people increases by 50%. Bonding with plasma proteins is 97%. Metabolized in the liver to form the active metabolite descarboethoxyloratadine with the participation of cytochrome isoenzymes CYP3A4 and, to a lesser extent, CYP2D6. Does not penetrate the blood-brain barrier. Excreted by the kidneys and bile. In patients with chronic renal failure and during hemodialysis, the pharmacokinetics remain virtually unchanged.
Cmax of loratadine in blood plasma is achieved after 3.28±1.25 hours and is 1.85±0.95 ng/ml, T1/2 is 11.29±5.52 hours.
Special instructions
Special instructions
Duration of use – no more than 5 days.
Do not use in the presence of metastatic tumors.
It is necessary to carry out laboratory monitoring of blood parameters.
Persons prone to drinking ethanol should consult a doctor before starting treatment with the drug, since paracetamol can have a damaging effect on the liver.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
Taking into account the possible development of side effects (dizziness, drowsiness) during the treatment period, it is not recommended to drive vehicles or engage in other activities that require increased concentration and high speed of psychomotor reactions.
Active ingredient
Active ingredient
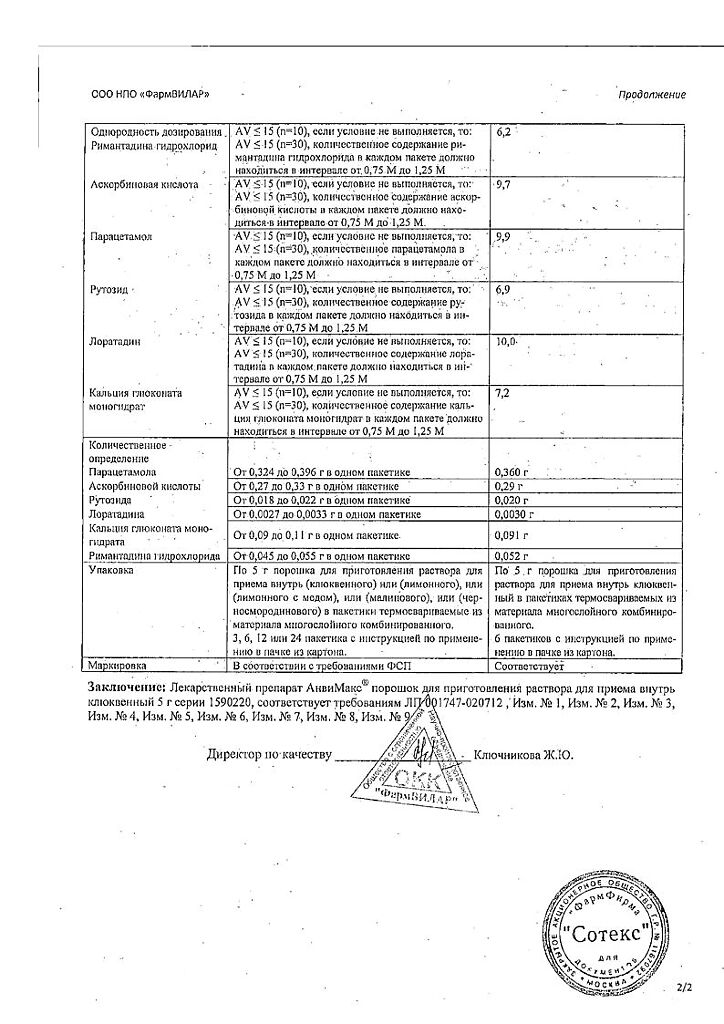
Paracetamol, Ascorbic acid, Rimantadine, Rutoside, Loratadine, Calcium gluconate
Composition
Composition
Active ingredients:
paracetamol – 360 mg,
ascorbic acid – 300 mg,
calcium gluconate monohydrate – 100 mg,
rimantadine hydrochloride – 50 mg,
rutoside trihydrate (in terms of rutoside) – 20 mg,
loratadine – 3 mg;
excipients:
aspartame – 30 mg,
hypromellose – 10 mg,
colloidal silicon dioxide – 20 mg,
lactose monohydrate – 4086 mg,
food flavoring (cranberry) – 21 mg.
Pregnancy
Pregnancy
Use during pregnancy and breastfeeding is contraindicated.
Use in children under 18 years of age is contraindicated.
Contraindications
Contraindications
Hypersensitivity to one or more components included in the drug; erosive and ulcerative lesions of the gastrointestinal tract (GIT) in the acute phase; gastrointestinal bleeding; hemophilia; hemorrhagic diathesis; hypoprothrombinemia; portal hypertension; vitamin deficiency K; renal failure; pregnancy, breastfeeding period; diseases of the thyroid gland, acute diseases of the kidneys, liver (acute glomerulonephritis, acute pyelonephritis, acute hepatitis, or exacerbation of chronic diseases of these organs); chronic alcoholism; hypercalcemia, severe hypercalciuria, nephrourolithiasis, sarcoidosis, simultaneous use of cardiac glycosides (risk of arrhythmias); lactose intolerance, lactase deficiency, glucose-galactose malabsorption; phenylketonuria.
Children under 18 years of age.
With caution
Restriction of use for epilepsy, cerebral atherosclerosis, diabetes mellitus, glucose-6-phosphate dehydrogenase deficiency, hemochromatosis, sideroblastic anemia, thalassemia, hyperoxaluria, nephrolithiasis, dehydration, electrolyte disturbances (risk of hypercalcemia), diarrhea, malabsorption syndrome, calcium nephrourolithiasis (history), hypercalciuria.
Elderly patients with arterial hypertension (the risk of hemorrhagic stroke increases due to rimantadine, which is part of the drug).
Side Effects
Side Effects
Possible undesirable side reactions are indicated in accordance with the components included in the composition.
From the central nervous system: increased excitability, drowsiness, tremor, hyperkinesia, dizziness, headache, flushing of the face.
From the digestive system: damage to the mucous membrane of the stomach and duodenum, dyspepsia, dry mucous membrane in the mouth, lack of appetite, bloating (flatulence), diarrhea (diarrhea).
From the urinary system: moderate pollakiuria.
From the hematopoietic organs: changes in blood parameters.
Allergic reactions: angioedema, anaphylactic shock, skin rash, itching, urticaria.
Skin: Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome) and acute generalized exanthematous pustulosis.
Other: inhibition of the function of the pancreatic insular apparatus (hyperglycemia, glycosuria).
Post-registration experience
During the use of the drug AnviMax®, cases of angioedema, lightheadedness, fever, decreased blood pressure, urticaria, itching, erythema, hearing loss, and sore throat have been described.
If any of the side effects indicated in the instructions get worse, or you notice any other side effects not listed in the instructions, tell your doctor immediately.
Interaction
Interaction
Paracetamol reduces the effectiveness of uricosuric drugs. Concomitant use of paracetamol in high doses increases the effect of anticoagulant drugs. Inducers of microsomal oxidation in the liver (phenytoin, barbiturates, rifampicin, phenylbutazone, tricyclic antidepressants), ethanol and hepatotoxic drugs increase the production of hydroxylated active metabolites, which makes it possible to develop severe intoxications even with a small overdose. When used simultaneously with metoclopramide, it is possible to increase the rate of absorption of paracetamol. Long-term use of barbiturates reduces the effectiveness of paracetamol. Microsomal oxidation inhibitors reduce the risk of hepatotoxicity.
Rimantadine enhances the stimulating effect of caffeine. Cimetidine reduces the clearance of rimantadine by 18%.
Ascorbic acid increases the concentration of benzylpenicillin in the blood. Improves the absorption of iron preparations in the intestines (converts ferric iron to divalent iron); may increase iron excretion when used concomitantly with deferoxamine. Increases the risk of developing crystalluria during treatment with salicylates and short-acting sulfonamides, slows down the excretion of acids by the kidneys, and increases the excretion of drugs that have an alkaline reaction (including alkaloids). Reduces the blood concentration of oral contraceptives. Increases the overall clearance of ethanol, which in turn reduces the concentration of ascorbic acid in the body. When used simultaneously, it reduces the chronotropic effect of isoprenaline. Barbiturates and primidone increase the excretion of ascorbic acid in the urine. Reduces the therapeutic effect of antipsychotic drugs (neuroleptics) – phenothiazine derivatives, tubular reabsorption of amphetamine and tricyclic antidepressants.
Loratadine. Inhibitors of CYP3A4 and CYP2D6 increase the concentration of loratadine in the blood.
Overdose
Overdose
Symptoms: during the first 24 hours after administration – pallor of the skin, nausea, diarrhea, vomiting, pain in the epigastric region; impaired glucose metabolism, metabolic acidosis (including lactic acidosis), hypokalemia, tachycardia, arrhythmia, headache, exacerbation of concomitant chronic diseases. Symptoms of liver dysfunction may appear 12-48 hours after an overdose. In case of severe overdose – liver failure with progressive encephalopathy, coma; acute renal failure with tubular necrosis (including in the absence of severe liver damage).
The threshold for overdose may be lowered in elderly patients, in patients taking certain drugs (eg, liver microsomal enzyme inducers), alcohol, or who are malnourished.
Treatment: administration of SH-group donors and precursors for the synthesis of glutathione – methionine within 8-9 hours after an overdose and acetylcysteine - within 8 hours. Gastric lavage, symptomatic therapy. The need for additional therapeutic measures (further administration of methionine, acetylcysteine) is determined depending on the concentration of paracetamol in the blood, as well as on the time elapsed after its administration.
Storage conditions
Storage conditions
In a dry place at a temperature not exceeding 25°C.
Keep out of the reach of children.
Shelf life
Shelf life
30 months.
Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
PharmVilar NPO LLC, Russia
Additional information
| Shelf life | 30 months. Do not use after the expiration date printed on the package. |
|---|---|
| Conditions of storage | In a dry place at a temperature not exceeding 25°C. Store out of the reach of children. |
| Manufacturer | PharmVilar NGO, Russia |
| Medication form | Powder for preparation of solution for oral administration |
| Brand | PharmVilar NGO |
Other forms…
Related products
Buy AnviMax, 5 g cranberry 6 pcs with delivery to USA, UK, Europe and over 120 other countries.