No products in the cart.
Angelique, 28 pcs.
€46.01 €39.87
Description
Pharmacodynamics
An anti-climacteric drug.
Anzhelik® contains estrogen, estradiol, which is identical to natural 17β-estradiol. Angelik® also contains the spironolactone derivative drospirenone which has gestagenic, antigonadotropic and antiandrogenic as well as anti-mineralocorticoid effects.
. Angelik® is a combination drug for hormone replacement therapy (HRT) for menopausal disorders in the postmenopausal period (natural menopause, hypogonadism, castration or premature ovarian exhaustion), including vasomotor symptoms (such as hot flashes, increased sweating), sleep disorders, decreased mood, irritability, atrophic changes of the urogenital tract in women with a failed uterus. Continuous hormone replacement therapy with Anjelik® avoids regular withdrawal bleeding, which is observed with cyclic or phasic ZGT.
Estradiol replenishes estrogen deficiency in women after menopause and provides effective treatment of psycho-emotional and vegetative menopausal symptoms (such as hot flashes, increased sweating, sleep disorders, increased nervous excitability, irritability, palpitations, cardialgia, dizziness, headache, libido reduction, muscle and joint pains); Involution of the skin and mucous membranes, especially the genitourinary system (urinary incontinence, dryness and irritation of the vaginal mucosa, painful intercourse).
Estradiol prevents loss of bone mass caused by estrogen deficiency, which is mainly related to suppression of osteoclast function and a shift of bone remodeling process towards bone formation. Long-term use of MHT has been shown to reduce the risk of periprosthetic bone fractures in postmenopausal women. When MHT is withdrawn, the rate of bone mass decline is comparable to that of the immediate postmenopausal period. It has not been shown that MHT can restore bone mass to premenopausal levels.
HST also has beneficial effects on the collagen content of the skin, as well as its density, and can also slow the formation of lines and wrinkles. In addition, because of the anti-androgenic properties of drospirenone, Angelik® has a therapeutic effect on androgen-dependent conditions such as acne, seborrhea, androgenic alopecia.
Drospirenone has anti-mineralocorticoid activity and increases sodium and water excretion, which may prevent high blood pressure, weight gain, edema, breast pain, and other symptoms associated with fluid retention. After 12 weeks of Anjelik® use, a slight decrease of BP (systolic BP decreased by 2-4 mmHg on average, diastolic BP decreased by 1-3 mmHg) was observed. The effect on BP was more pronounced in women with arterial hypertension. After 12 months of using Angelik® the average body weight remained unchanged or decreased by 1.1-1.2 kg.
Drospirenone is devoid of any androgenic, estrogenic, glucocorticosteroid and antiglucocorticosteroid activity and has no effect on glucose tolerance and insulin resistance. This, in combination with anti-mineralocorticoid and anti-androgenic activity, provides drospirenone with a biochemical and pharmacological profile similar to that of natural progesterone.
The administration of Angelik® leads to a decrease in total and LDL cholesterol and a slight increase in triglyceride levels. Drospirenone reduces the increase in triglyceride concentration caused by estradiol.
The addition of drospirenone prevents the development of hyperplasia and endometrial cancer.
Observational studies suggest that among postmenopausal women the incidence of colorectal cancer decreases with the use of ZGT. The mechanism of action is still unclear.
Pharmacokinetics
Estradiol
Estradiol absorption
After oral administration, estradiol is quickly and completely absorbed from the gastrointestinal tract. It undergoes a “first pass” effect with the formation of estrone, estriol and estrone sulfate. Bioavailability when ingested is about 5% and does not depend on food intake. The Cmax of estradiol in serum is approximately 22 pg/ml and is reached after 6-8 hours. The bioavailability of estradiol is not affected by food intake.
Distribution
Bound to albumin and sex steroid-binding globulin (SBSG). The free fraction of estradiol in serum is approximately 1-12% and GSSB bound 40-45%. The apparent Vd after a single IV administration is about 1 L/kg. After multiple administration, estradiol concentrations are approximately 2 times higher than after single administration, with Css ranging from 20 pg/ml to 43 pg/ml. After discontinuation, estradiol and estrone levels return to baseline values within approximately 5 days.
Metabolism
. Estradiol is metabolized mainly in the liver, partially – in the intestine, kidneys, skeletal muscles and in the target organs to form estrone, estriol, catecholestrogens, and sulfate and glucuronide conjugates of these compounds, which have significantly lower estrogenic activity compared to estradiol or are inactive at all.
The serum clearance of estradiol is about 30 ml/min/kg. Estradiol metabolites are excreted in the urine and bile. The T1/2 is approximately 24 hours.
Drospirenone
Intake
Drospirenone is rapidly and completely absorbed from the gastrointestinal tract after oral administration. Bioavailability is 76-85% and does not depend on food intake. Food intake does not affect the bioavailability of drospirenone.
Distribution
After a single or multiple doses of 2 mg, serum Cmax is reached after 1 hour and is about 22 ng/ml. Thereafter, there is a biphasic decrease in the serum concentration of drospirenone with a final T1/2 of approximately 35-39 h. drospirenone binds to albumin and does not bind to HSPC and corticoid-binding globulin (CRB); about 3-5% is the free fraction. Due to the long T1/2 Css is reached after 10 days of daily administration of Angelik® and exceeds the concentration after a single dose by 2-3 times.
Metabolism
The main metabolites are the acidic form of drospirenone and 4,5-dihydro-drospirenone-3-sulfate, which are formed without participation of cytochrome P450 isoenzymes.
The serum clearance of drospirenone is 1.2-1.5 ml/min/kg. Some part of the dose received is excreted unchanged. Most of the dose is excreted by the kidneys and through the intestine as metabolites at a ratio of 1.2:1.4; T1/2 is about 40 hours.
Indications
Indications
Hormone replacement therapy for menopausal disorders in postmenopause.
Prevention of postmenopausal osteoporosis.
Pharmacological effect
Pharmacological effect
Pharmacodynamics
Anticlimacteric drug.
The drug Angelique® contains estrogen – estradiol, which is identical to natural 17β-estradiol. Also, the drug Angeliq® includes a spironolactone derivative – drospirenone, which has a gestagenic, antigonadotropic and antiandrogenic, as well as antimineralocorticoid effect.
Angeliq® is a combination drug for hormone replacement therapy (HRT) for menopausal disorders in the postmenopausal period (natural menopause, hypogonadism, castration or premature ovarian failure), including vasomotor symptoms (such as hot flashes, increased sweating), sleep disturbances, decreased mood, irritability, atrophic changes in the genitourinary tract in women with unremoved uterus. Continuous hormone replacement therapy with Angeliq® avoids regular withdrawal bleeding, which is observed with cyclic or phase HRT.
Estradiol replenishes the deficiency of estrogen in the female body after menopause and provides effective treatment of psycho-emotional and autonomic menopausal symptoms (such as hot flashes, increased sweating, sleep disturbances, increased nervous excitability, irritability, palpitations, cardialgia, dizziness, headache, decreased libido, muscle and joint pain); involution of the skin and mucous membranes, especially the genitourinary system (urinary incontinence, dryness and irritation of the vaginal mucosa, pain during sexual intercourse).
Estradiol prevents bone loss caused by estrogen deficiency, which is mainly associated with suppression of osteoclast function and a shift in the process of bone remodeling towards bone formation. Long-term use of HRT has been shown to reduce the risk of peripheral bone fractures in women after menopause. When HRT is discontinued, the rate of bone mass decline is comparable to that characteristic of the period immediately after menopause. It has not been proven that HRT can restore bone mass to premenopausal levels.
HRT also has a beneficial effect on the collagen content of the skin, as well as its density, and may also slow down the formation of wrinkles. In addition, due to the antiandrogenic properties of drospirenone, Angeliq® has a therapeutic effect on androgen-dependent diseases such as acne, seborrhea, androgenic alopecia.
Drospirenone has antimineralocorticoid activity, increases the excretion of sodium and water, which can prevent increased blood pressure, weight gain, edema, breast tenderness and other symptoms associated with fluid retention. After 12 weeks of using the drug Angeliq®, a slight decrease in blood pressure was observed (systolic – on average by 2-4 mm Hg, diastolic – by 1-3 mm Hg). The effect on blood pressure was more pronounced in women with arterial hypertension. After 12 months of using the drug Angeliq®, the average body weight remained unchanged or decreased by 1.1-1.2 kg.
Drospirenone is devoid of any androgenic, estrogenic, glucocorticosteroid and anti-glucocorticosteroid activity and does not affect glucose tolerance or insulin resistance. This, combined with its antimineralocorticoid and antiandrogenic effects, gives drospirenone a biochemical and pharmacological profile similar to natural progesterone.
Taking Angeliq® leads to a decrease in total cholesterol and LDL cholesterol, as well as a slight increase in triglyceride levels. Drospirenone reduces the increase in triglyceride concentrations caused by estradiol.
The addition of drospirenone prevents the development of endometrial hyperplasia and cancer.
Observational studies suggest that among postmenopausal women, the incidence of colon cancer is reduced when using HRT. The mechanism of action is still unclear.
Pharmacokinetics
Estradiol
Suction
After taking the drug orally, estradiol is quickly and completely absorbed from the gastrointestinal tract. Subjects to a “first pass” effect with the formation of estrone, estriol and estrone sulfate. Bioavailability when taken orally is about 5% and is independent of food intake. Cmax of estradiol in serum is approximately 22 pg/ml and is achieved after 6-8 hours. Food intake does not affect the bioavailability of estradiol.
Distribution
Binds to albumin and sex steroid binding globulin (SGBS). The free fraction of estradiol in serum is approximately 1-12%, and bound SHPS is 40-45%. The apparent Vd after a single intravenous injection is about 1 l/kg. After repeated use, the concentration of estradiol is approximately 2 times higher than after a single dose, while Css varies from 20 pg/ml to 43 pg/ml. After discontinuation of use, levels of estradiol and estrone return to baseline values within approximately 5 days.
Metabolism
Estradiol is metabolized primarily in the liver, partially in the intestines, kidneys, skeletal muscles and target organs with the formation of estrone, estriol, catechol estrogens, as well as sulfate and glucuronide conjugates of these compounds, which have significantly less estrogenic activity compared to estradiol or are completely inactive.
Removal
Serum clearance of estradiol is about 30 ml/min/kg. Estradiol metabolites are excreted in urine and bile. T1/2 is approximately 24 hours.
Drospirenone
Suction
After oral administration, drospirenone is quickly and completely absorbed from the gastrointestinal tract. Bioavailability is 76-85% and does not depend on food intake. Food intake does not affect the bioavailability of drospirenone.
Distribution
After a single or multiple dose of 2 mg, Cmax in serum is reached after 1 hour and is about 22 ng/ml. After this, a biphasic decrease in the concentration of drospirenone in the serum is observed with a final half-life of about 35-39 hours. Drospirenone binds to albumin and does not bind to SHBG and corticoid-binding globulin (CBG); about 3-5% is the free fraction. Due to the long T1/2 Css is achieved after 10 days of daily use of the drug Angeliq® and exceeds the concentration after a single dose by 2-3 times.
Metabolism
The main metabolites are the acid form of drospirenone and 4,5-dihydro-drospirenone-3-sulfate, which are formed without the participation of isoenzymes of the cytochrome P450 system.
Removal
The serum clearance of drospirenone is 1.2-1.5 ml/min/kg. Some part of the received dose is excreted unchanged. Most of the dose is excreted by the kidneys and through the intestines in the form of metabolites in a ratio of 1.2:1.4; T1/2 – about 40 hours.
Special instructions
Special instructions
Angeliq® is not used for contraception.
If contraception is necessary, non-hormonal methods should be used (with the exception of calendar and temperature methods). If you suspect pregnancy, you should stop taking the pills until pregnancy has been ruled out.
If any of the following conditions or risk factors are present or worsening, the individual risk-benefit ratio of treatment should be assessed before starting or continuing to take Angeliq.
When prescribing HRT to women who have several risk factors for the development of thrombosis or a high degree of severity of one of the risk factors, the possibility of mutually enhancing the effect of risk factors and the prescribed treatment on the development of thrombosis should be taken into account. In such cases, the total value of the existing risk factors increases. If there is a high risk, Angeliq® is contraindicated.
Venous thromboembolism
A number of controlled randomized as well as epidemiological studies have revealed an increased relative risk of developing venous thromboembolism (VTE) during HRT, i.e. deep vein thrombosis or pulmonary embolism. Therefore, when prescribing Angeliq® to women with risk factors for VTE, the risk-benefit ratio of treatment should be carefully weighed and discussed with the patient.
Risk factors for developing VTE include individual and family history (the presence of VTE in first-degree relatives at a relatively young age may indicate a genetic predisposition) and severe obesity.
The risk of VTE also increases with age. The possible role of varicose veins in the development of VTE remains controversial. The risk of VTE may temporarily increase with prolonged immobilization, “major” elective and trauma surgeries, or major trauma. Depending on the cause or duration of immobilization, the question of the advisability of temporarily stopping taking the drug Angeliq should be decided.
Treatment should be stopped immediately if symptoms of thrombotic disorders appear or if their occurrence is suspected.
Arterial thromboembolism
Randomized controlled trials with long-term use of conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA) did not provide evidence of a beneficial effect on the cardiovascular system. In large-scale clinical trials of this compound, a possible increase in the risk of coronary heart disease in the first year of use was identified, followed by a lack of beneficial effect. One large clinical trial using CLE alone found a potential reduction in the incidence of CAD among women aged 50–59 years, but no overall benefit in the overall study population. As a secondary outcome, two large clinical trials using CLE as monotherapy or in combination with MPA found a 30-40% increase in the risk of stroke. It is therefore unknown whether this increased risk applies to HRT products containing other types of estrogens and progestogens or to non-oral routes of administration.
Endometrial cancer
With long-term estrogen monotherapy, the risk of developing endometrial hyperplasia or carcinoma increases. Studies have confirmed that the addition of gestagens reduces the risk of endometrial hyperplasia and cancer.
Breast cancer
Clinical trial data and observational studies have found an increase in the relative risk of developing breast cancer in women using HRT for several years. This may be due to earlier diagnosis, accelerated growth of an existing tumor during HRT, or a combination of both factors.
The relative risk increases with duration of therapy but may be absent or reduced with estrogen-only treatment. This increase is comparable to the increased risk of breast cancer in women with a later onset of natural menopause, as well as with obesity and alcohol abuse. The increased risk gradually decreases to normal levels within a few (but most of five) years after stopping HRT.
The increased risk of breast cancer has been suggested based on the results of more than 50 epidemiological studies (risk ranges from 1 to 2).
Two large randomized trials of CLE alone or chronically combined with MPA reported estimated risk ratios of 0.77 (95% confidence interval: 0.59–1.01) or 1.24 (95% confidence interval: 1.01–1.54) after approximately 6 years of HRT use. It is unknown whether this increased risk also applies to other HRT products.
HRT increases mammographic breast density, which in some cases may have a negative effect on X-ray detection of breast cancer.
Liver tumor
During the use of sex steroids, which include drugs for HRT, in rare cases benign, and even more rarely, malignant liver tumors were observed. In some cases, these tumors have resulted in life-threatening intra-abdominal bleeding. If there is pain in the upper abdomen, an enlarged liver, or signs of intra-abdominal bleeding, the differential diagnosis should take into account the possibility of a liver tumor.
Gallstone disease
It is known that estrogens increase the lithogenicity of bile. Some women are predisposed to developing gallstones when treated with estrogen.
Dementia
There is limited clinical trial data on a possible increased risk of dementia in women starting CLE-containing medications aged 65 years or older. As observed in studies, the risk may be reduced if HRT drugs containing CLE are started in early menopause. It is not known whether this applies to other HRT medications.
Other states
Treatment should be stopped immediately if migraine-like or frequent and unusually severe headaches appear for the first time, as well as if other symptoms appear – possible precursors of a thrombotic stroke of the brain.
The relationship between HRT and the development of clinically significant arterial hypertension has not been established. A slight increase in blood pressure has been described in women taking HRT; clinically significant increases are rare. However, in some cases, if persistent clinically significant arterial hypertension develops while taking HRT, discontinuation of HRT may be considered. In women with high blood pressure, there may be a slight decrease in blood pressure while taking the drug Angeliq®. In women with normal blood pressure, significant changes in blood pressure are not expected.
In renal failure, the ability to excrete potassium may be reduced. Taking drospirenone does not affect serum potassium concentrations in patients with mild to moderate forms of renal failure. The risk of developing hyperkalemia cannot theoretically be excluded only in the group of patients whose serum potassium concentration before treatment was determined at ULN and who additionally take potassium-sparing drugs.
For mild liver dysfunction, incl. Various forms of hyperbilirubinemia, such as Dubin-Johnson syndrome or Rotor syndrome, require medical supervision, as well as periodic liver function tests. If liver function indicators deteriorate, Angeliq® should be discontinued.
In case of recurrence of cholestatic jaundice or cholestatic itching, which was observed for the first time during pregnancy or previous treatment with sex steroid hormones, taking Angeliq should be stopped immediately.
Special monitoring of women is necessary when triglyceride concentrations increase. In such cases, the use of HRT may cause a further increase in the concentration of triglycerides in the blood, which increases the risk of acute pancreatitis.
Although HRT may affect peripheral insulin resistance and glucose tolerance, there is usually no need to change the treatment regimen of diabetic patients when undergoing HRT. However, women with diabetes should be monitored when undergoing HRT.
Some patients under the influence of HRT may develop undesirable manifestations of estrogen stimulation, for example, abnormal uterine bleeding. Frequent or persistent pathological uterine bleeding during treatment is an indication for examination of the endometrium in order to exclude an organic disease.
Under the influence of estrogens, uterine fibroids can increase in size. In this case, treatment should be stopped.
It is recommended to discontinue treatment if endometriosis relapses during HRT.
If prolactinoma is suspected, this disease should be excluded before starting treatment. If prolactinoma is detected, the patient should be under close medical supervision (including periodic assessment of drug concentrations).
In some cases, chloasma may occur, especially in women with a history of chloasma during pregnancy. During treatment with Angeliq®, women prone to chloasma should avoid prolonged exposure to the sun or ultraviolet radiation.
The following conditions may occur or be aggravated by HRT, and women with these conditions should be under medical supervision when undergoing HRT: epilepsy, benign breast tumor, bronchial asthma, migraine, porphyria, otosclerosis, systemic lupus erythematosus, chorea minor.
In women with hereditary forms of angioedema, exogenous estrogens may cause or worsen symptoms of angioedema.
Additional information
There is no data on the need for dose adjustment in women under 65 years of age. When using the drug Angeliq® in women over 65 years of age, the information presented in the subsection “Dementia” should be taken into account.
Drospirenone is well tolerated in women with mild or moderate hepatic impairment.
In women with mild to moderate renal impairment, a slight slowdown in the elimination of drospirenone was observed, which was not clinically significant.
Preclinical Safety Data Preclinical data obtained from routine repeated-dose toxicity, genotoxicity, carcinogenicity and reproductive toxicity studies do not indicate a particular risk to humans. However, it should be remembered that sex steroids can promote the growth of certain hormone-dependent tissues and tumors.
Medical examination
Before starting or resuming taking Angeliq®, you should review the patient’s medical history in detail and conduct a physical and gynecological examination. The frequency and nature of such examinations should be based on existing standards of medical practice, taking into account the individual characteristics of each patient (but not less than once every 6 months) and should include blood pressure measurement, assessment of the condition of the mammary glands, abdominal and pelvic organs, including cytological examination of the cervical epithelium.
In the presence of prolactinoma, periodic determination of prolactin concentration is required.
Impact on laboratory results
Taking sex steroids can affect the biochemical parameters of the liver, thyroid gland, adrenal glands and kidneys, the plasma content of transport proteins, such as globulin that binds sex hormones and lipid/lipoprotein fractions, indicators of carbohydrate metabolism, coagulation and fibrinolysis. Angeliq® does not have a negative effect on glucose tolerance.
Impact on the ability to drive vehicles and other mechanisms that require increased concentration
Not identified.
Active ingredient
Active ingredient
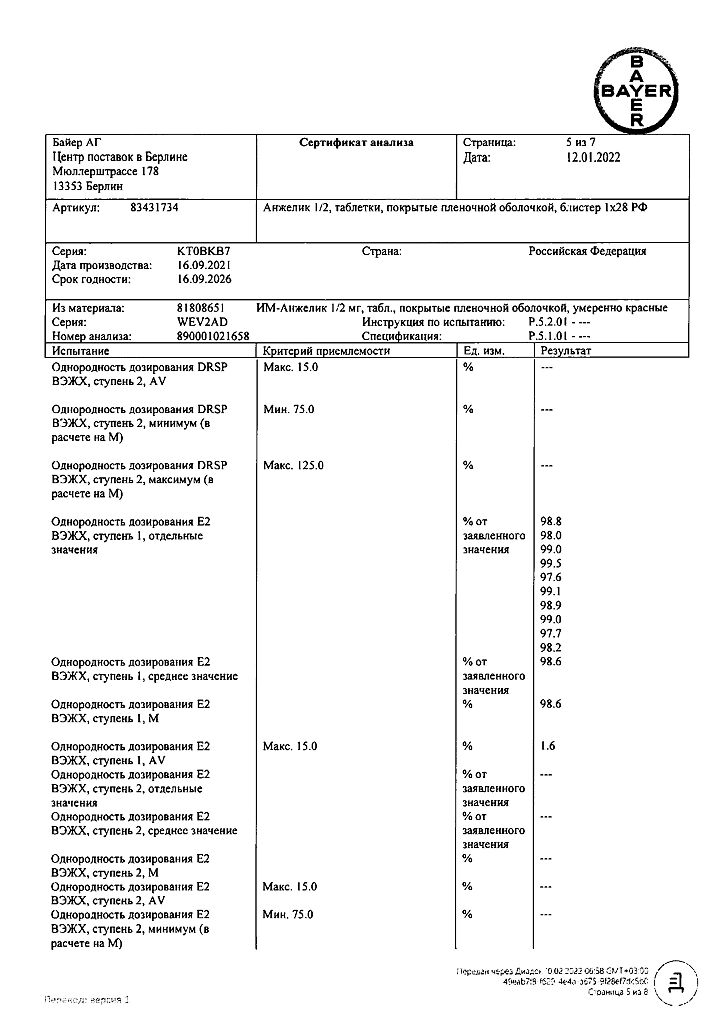
Drospirenone, Estradiol
Composition
Composition
Active ingredients:
estradiol (in the form of hemihydrate) 1 mg;
drospirenone 2 mg.
Excipients:
lactose monohydrate – 48.2 mg,
corn starch – 14.4 mg,
pregelatinized corn starch – 9.6 mg,
povidone K25 – 4 mg,
magnesium stearate – 0.8 mg,
hypromellose – 1.0112 mg,
macrogol 6000 – 0.2024 mg,
talc – 0.2024 mg,
titanium dioxide – 0.5438 mg,
red iron oxide dye – 0.0402 mg.
Pregnancy
Pregnancy
HRT is contraindicated during pregnancy and breastfeeding.
If pregnancy is detected while taking Angeliq®, the drug should be discontinued immediately.
Small amounts of sex hormones can be excreted in breast milk.
Contraindications
Contraindications
It is not recommended to start taking Angeliq® if you have any of the following conditions; If any of these conditions occur while taking Angeliq®, you should immediately stop using the drug:
hypersensitivity to the components of the drug Angeliq®;
vaginal bleeding of unknown etiology;
confirmed or suspected breast cancer or a history of breast cancer;
confirmed or suspected diagnosis of a hormone-dependent precancerous disease or a hormone-dependent malignant tumor;
benign or malignant liver tumors (including a history);
severe liver disease;
severe kidney disease currently or in history or acute renal failure (until normalization of renal function indicators);
acute arterial thrombosis or thromboembolism (including those leading to myocardial infarction, stroke);
deep vein thrombosis in the acute stage, venous thromboembolism currently or in history;
presence of a high risk of venous and arterial thrombosis;
pulmonary embolism;
severe hypertriglyceridemia;
pregnancy and lactation;
children and adolescents up to 18 years of age;
congenital lactase deficiency, lactose intolerance, glucose-galactose malabsorption.
Angeliq® should be prescribed with caution in the following diseases: arterial hypertension, congenital hyperbilirubinemia (Gilbert, Dubin-Johnson and Rotor syndromes), cholestatic jaundice or cholestatic pruritus during previous pregnancy, endometriosis, uterine fibroids, diabetes mellitus.
It must be taken into account that estrogens alone or in combination with gestagens should be used with caution in the following diseases and conditions: smoking, hypercholesterolemia, obesity, systemic lupus erythematosus, dementia, gallbladder diseases, retinal vascular thrombosis, moderate hypertriglyceridemia, edema in chronic heart failure, severe hypocalcemia, endometriosis, bronchial asthma, epilepsy, migraine, porphyria, liver hemangiomas, hyperkalemia, conditions predisposing to the development of hyperkalemia, taking drugs that cause hyperkalemia (potassium-sparing diuretics, potassium supplements, ACE inhibitors, angiotensin II receptor antagonists and heparin).
Side Effects
Side Effects
The most common adverse drug reactions observed with the use of Angeliq® were breast tenderness, bleeding from the genital tract, gastrointestinal pain and abdominal pain. These reactions occur in ≥ 6% of women using Angeliq.
Irregular bleeding usually disappears with long-term therapy. The frequency of bleeding decreases with increasing duration of treatment.
Serious adverse reactions include arterial and venous thromboembolic complications and breast cancer.
Adverse drug reactions described in clinical studies using the drug Angeliq® are presented in order of decreasing severity. The incidence of adverse events was classified as follows:
very often (≥1/10);
often (≥1/10, <1/10);
uncommon (≥1/1000, <1/100);
rare (<1/10,000).
Mental disorders: often – emotional lability.
From the side of the central nervous system: often – migraine.
From the cardiovascular system: infrequently – venous and arterial thromboembolic complications (occlusion of peripheral deep veins, thrombosis and embolism/occlusion of pulmonary vessels, thrombosis, embolism and infarction/myocardial infarction/cerebral infarction and stroke, with the exception of hemorrhagic).
From the digestive system: often – gastrointestinal pain, abdominal pain.
From the reproductive system: very often – pain in the mammary glands (including discomfort in the mammary glands), bleeding from the genital tract; often – cervical polyp; infrequently – breast cancer*.
* – Data on the relationship with the use of the drug were obtained from post-marketing observations; Frequency data are obtained from clinical studies using the drug Angeliq®.
For more information about venous and arterial thromboembolic complications, breast cancer and migraine, see “Contraindications” and “Special Instructions”.
Adverse reactions that occur in isolated cases, or the symptoms of which develop a very long time after the start of therapy and which are considered associated with the use of drugs from the group of combined drugs for continuous HRT: liver tumors (benign and malignant); hormone-dependent malignant tumors or hormone-dependent precancerous diseases (if it is known that the patient has such conditions, this is a contraindication to the use of Angeliq®); cholelithiasis; dementia; endometrial cancer; arterial hypertension; liver dysfunction; hypertriglyceridemia; changes in glucose tolerance or effects on peripheral tissue insulin resistance; an increase in the size of uterine fibroids; reactivation of endometriosis; prolactinoma; chloasma; jaundice and/or itching associated with cholestasis.
The emergence or worsening of conditions for which the relationship with the use of HRT has not been clearly proven: epilepsy; benign diseases of the mammary glands; bronchial asthma; porphyria; systemic lupus erythematosus; otosclerosis, minor chorea.
In women with hereditary angioedema, exogenous estrogens may exacerbate symptoms.
Hypersensitivity reactions (including symptoms such as rash and urticaria) have also been observed.
For additional information about serious adverse events associated with hormone replacement therapy, see “Special Instructions.”
Interaction
Interaction
Long-term treatment with drugs that induce liver enzymes (for example, some anticonvulsants and antimicrobial drugs) may increase the clearance of sex hormones and reduce their clinical effectiveness.
A similar property – to induce liver enzymes – was found in hydantoins, barbiturates, primidone, carbamazepine and rifampicin, the presence of this feature is also suggested in oxcarbazepine, topiramate, felbamate and griseofulvin.
Maximum enzyme induction is usually observed no earlier than 2-3 weeks, but it may then persist for at least 4 weeks after discontinuation of the drug.
In rare cases, a decrease in estradiol levels has been observed during concomitant use of certain antibiotics (for example, penicillins and tetracyclines).
The main metabolites of drospirenone are formed in plasma without the participation of the cytochrome P450 system. Therefore, the effect of inhibitors of the cytochrome P450 system on the metabolism of drospirenone is unlikely. However, CYP3A4 inhibitors (eg, cimetidine, ketoconazole) may inhibit the metabolism of estradiol.
Based on in vitro interaction studies, as well as in vivo studies in female volunteers taking omeprazole, simvastatin and midazolam as markers, it can be concluded that the effect of drospirenone 3 mg on the metabolism of other medicinal substances is unlikely.
The use of Angelica in women receiving antihypertensive therapy (for example, ACE inhibitors, angiotensin II receptor antagonists, hydrochlorothiazide) may slightly increase the antihypertensive effect.
An increase in serum potassium levels when taking Angelica in combination with NSAIDs or antihypertensive drugs is unlikely. The combined use of the above three types of drugs may lead to a slight increase in serum potassium levels, more pronounced in women with type 1 and type 2 diabetes mellitus.
Excessive alcohol consumption during HRT may increase circulating estradiol levels.
Overdose
Overdose
Acute toxicity studies have not revealed a risk of acute side effects when accidentally taking the drug in quantities many times higher than the daily therapeutic dose.
In clinical studies, the use of drospirenone up to 100 mg or combined estrogen/progestin drugs containing 4 mg estradiol was well tolerated.
Symptoms that may occur in case of overdose: nausea, vomiting, bleeding from the vagina.
Treatment: there is no specific antidote; if necessary, symptomatic therapy is carried out.
Storage conditions
Storage conditions
At room temperature no higher than 25 °C
Shelf life
Shelf life
5 years
Manufacturer
Manufacturer
Bayer Weimar GmbH & Co. KG, Germany
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At room temperature not higher than 25 °C |
| Manufacturer | Bayer Weimar GmbH & Co. KG, Germany |
| Medication form | pills |
| Brand | Bayer Weimar GmbH & Co. KG |
Related products
Gynecology and Obstetrics
Prepidil, intracervical gel 0.5 mg/3 g syringes with catheter
Buy Angelique, 28 pcs. with delivery to USA, UK, Europe and over 120 other countries.