No products in the cart.
Androcur-Depo, 100 mg/ml 3 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Pharmacodynamics
A long-acting antiandrogenic drug. Competitively binds to tissue androgen receptors. Reduces or completely eliminates the effect of androgens on the target organs (including the prostate). It has gestagenic activity and antigonadotropic properties.
When treating with Androcur Depo a decrease in libido and potency as well as a decrease in gonadal function is observed. These phenomena are reversible and go away after discontinuation of the drug.
Pharmacokinetics
Absorption and distribution
After the intravenous administration of Androcur Depot cyproterone acetate is slowly and completely released. Cmax is reached after 2-3 days and is 180±54 ng/mL. Thereafter, there is a decrease in plasma concentration of the drug with a T1/2 of 4±1.1 days. Css is reached after approximately 5 weeks of use of Androcur Depot.
Ciproterone acetate is almost completely bound to plasma proteins (albumin) (only 3.5-4% is free). Because the binding to plasma proteins is non-specific, changes in hGH (sex hormone-binding globulin) levels do not affect the pharmacokinetic parameters of cyproterone acetate.
Elimation
Most of the administered dose is excreted as metabolites in the urine and feces; a small amount is excreted unchanged in the bile.
Indications
Indications
Active ingredient
Active ingredient
Composition
Composition
Actives:
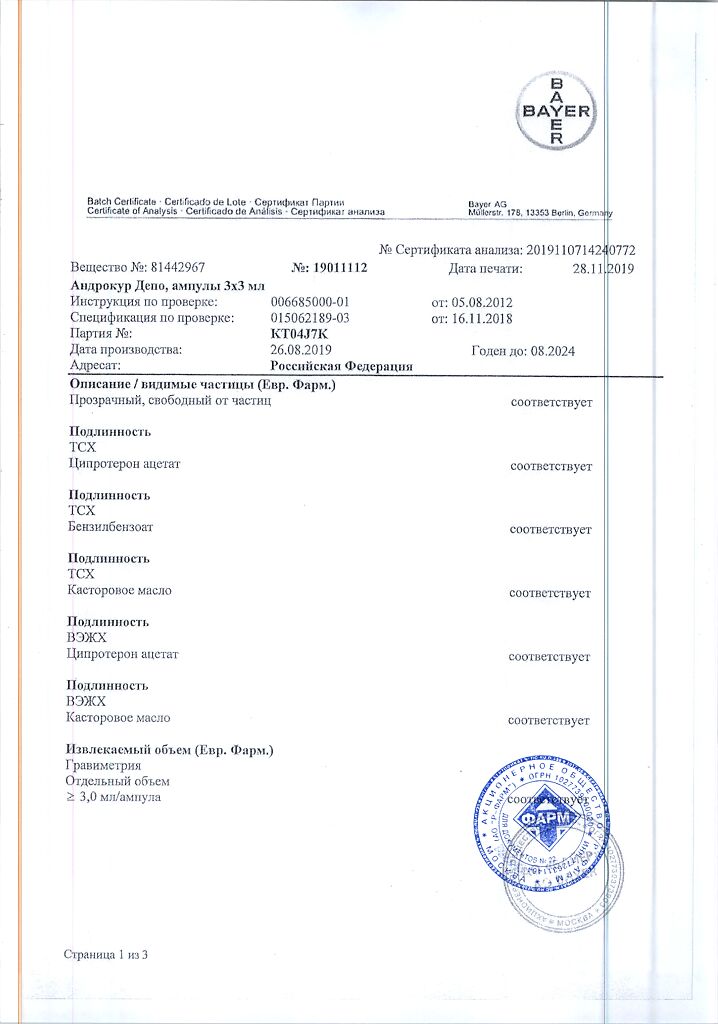
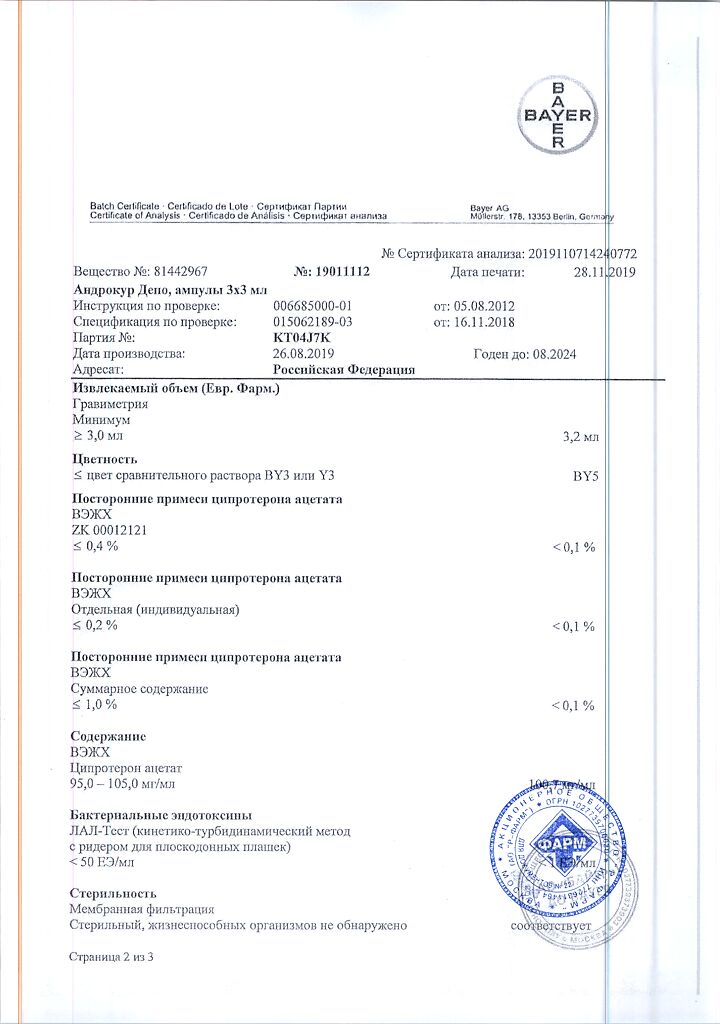
Cyproterone acetate 100 mg.
Excipients:
benzyl benzoate – 618.6 mg,
castor oil – 353.4 mg.
How to take, the dosage
How to take, the dosage
Androcur Depot is administered only deeply intramuscularly.
In order to reduce libido in men, the drug is administered every 10-14 days in a dose of 300 mg (1 ampoule). In exceptional cases, when this dose is insufficient, 600 mg (2 ampoules) may be injected every 10-14 days (preferably 3 ml in the right and left buttocks). If treatment is satisfactory, an attempt should be made to reduce the dose, gradually increasing the intervals between injections.
In order to stabilize the therapeutic effect, injections of Androcur Depot should be continued over a long period of time and, if possible, with simultaneous psychotherapeutic treatment.
In inoperable prostate cancer, 300 mg of the drug is administered to rule out the action of androgens of the adrenal cortex. If the condition improves or remission is achieved, treatment should not be interrupted or the dose reduced.
In rare cases, short-term reactions (urges to cough, episodes of coughing, feeling of shortness of breath) may occur during or immediately after the injection. Very slow administration of the drug avoids these reactions.
Interaction
Interaction
Concomitant use of Androcur Depot may alter the clinical effectiveness of oral hypoglycemic drugs or insulin.
Special Instructions
Special Instructions
The drug is not intended for use in women.
Pending Androcur Depot before puberty is undesirable because one cannot rule out the adverse effects of the drug on the patient’s growth and their still unstabilized endocrine system.
When using cyproterone acetate at a dose of 200-300 mg, direct hepatotoxic effects (development of jaundice, liver failure) have been described, which in several cases resulted in death. Most of the described observations refer to patients with prostate cancer. The toxic effects are dose-dependent, and usually develop only after a few months of treatment. Liver function should be investigated before the start of treatment, as well as if hepatotoxic effects are suspected. If toxic liver damage is confirmed, cyproterone acetate should be discontinued unless hepatotoxicity is due to other causes, such as liver metastasis. In this case, continuation of treatment is possible only when the expected benefit of treatment outweighs the risk.
In rare cases, liver tumors have developed during the use of sex steroids. If severe abdominal pain, liver enlargement, or signs of intra-abdominal bleeding occur, this should be considered when making a differential diagnosis.
Patients with diabetes mellitus are treated under constant medical supervision.
High doses of cyproterone acetate may on rare occasions produce a sensation of shortness of breath. In such cases when making a differential diagnosis it is necessary to take into account that progesterone and synthetic gestagens have a stimulating effect on breathing. This develops hypocapnia and associated compensatory metabolic alkalosis, which does not require treatment.
There have been isolated reports of thromboembolic events during treatment with Androcur Depot. However, the causal relationship with taking the drug seems doubtful.
The simultaneous use of alcohol, which has a disinhibiting effect, in patients with pathologically increased libido may decrease the effect of Androcur Depo therapy.
Control of laboratory parameters
Hepatic, adrenal and peripheral blood function should be monitored carefully before and during therapy.
Impact on ability to drive and operate vehicles and other mechanisms requiring increased attention
Patients engaged in potentially hazardous activities requiring increased attention should be aware that Androcur Depo may cause fatigue, decreased activity and impaired ability to concentrate.
Contraindications
Contraindications
The appropriateness of prescribing Androcur Depo to patients with prostate cancer with thromboembolic syndrome in the anamnesis, as well as in the presence of severe diabetes with angiopathy should be decided individually in each case, comparing the expected benefits of treatment and possible adverse effects.
Side effects
Side effects
Endocrine system disorders: when used for several weeks, Androcur Depot suppresses spermatogenesis and shows antigonadotropic properties (after discontinuation of the drug spermatogenesis is gradually restored over several months); sometimes – gynecomastia (in some patients – combined with increased tactile sensitivity and nipple pain), which gradually disappears after discontinuation of the drug; in some cases, body weight may change.
CNS disorders: possible feeling of fatigue, decreased activity; sometimes anxiety, depression.
Overdose
Overdose
The development of pathological effects in case of accidental overdose is unlikely.
Treatment: if necessary, symptomatic therapy is carried out.
Similarities
Similarities
Additional information
| Shelf life | 5 years. |
|---|---|
| Conditions of storage | Keep out of reach of children. |
| Manufacturer | Bayer AG, Germany |
| Medication form | solution |
| Brand | Bayer AG |
Related products
Buy Androcur-Depo, 100 mg/ml 3 pcs with delivery to USA, UK, Europe and over 120 other countries.