No products in the cart.
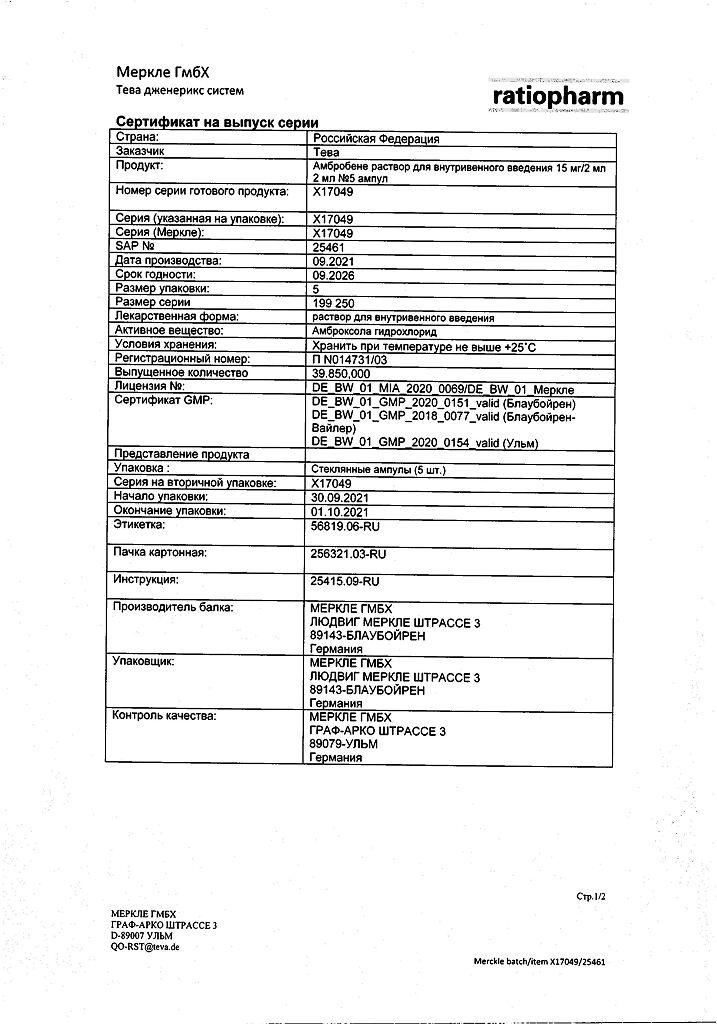
Ambrobene, 15 mg/2 ml 2 ml 5 pcs
€5.66 €5.03
Description
Pharmacotherapeutic group: expectorant mucolytic.
ATC code: R05CB06
Pharmacological action:
Pharmacodynamics:
Ambroxol is a benzylamine, a metabolite of bromhexine. It differs from bromhexin in the absence of a methyl group and the presence of a hydroxyl group in the para-trans position of the cyclohexyl ring. It has secretomotor, secretolytic and expectorant action.
Preclinical studies have shown that ambroxol stimulates serous gland cells of the bronchial mucosa. By activating cells of the ciliated epithelium and reducing sputum viscosity, it improves mucociliary transport.
Ambroxol activates surfactant formation with a direct effect on alveolar type 2 pneumocytes and small airway Clara cells.
Studies on cell cultures and in vivo animal studies have shown that ambroxol stimulates the formation and secretion of a substance (surfactant) active on the surface of the alveoli and bronchi of the embryo and adult.
An antioxidant effect of ambroxol has also been proven in preclinical studies. Ambroxol when used together with antibiotics amoxicycycline, cefuroxime, erythromycin and doxycycline increases their concentration in sputum and bronchial secretion.
Pharmacokinetics:
In parenteral administration, ambroxol rapidly penetrates tissues. The highest concentration is found in the lungs. The maximum concentration is reached after 1-3 hours.
The binding to plasma proteins is about 85% (80-90%). The plasma elimination half-life is 7 to 12 hours. The total half-life of ambroxol and its metabolites is approximately 22 hours.
It is excreted mainly by the kidneys as metabolites – 90%, less than 10% is excreted unchanged.
Given the high binding to plasma proteins, large volume of distribution and slow redistribution from tissues into blood, there is no significant excretion of ambroxol with dialysis or forced diuresis.
In patients with severe liver disease, ambroxol clearance is reduced by 20-40%.
Ambroxol penetrates the cerebrospinal fluid and through the placental barrier, and is excreted in breast milk.
Indications
Indications
The drug Ambrobene is used for the treatment of acute and chronic diseases of the respiratory tract, accompanied by impaired formation and discharge of sputum in adults and children from the first year of life.
Special instructions
Special instructions
Before using Ambrobene, consult your doctor or pharmacist. Tell your doctor if you have the following conditions:
impaired bronchial motor function and increased sputum production (with immotile cilia syndrome);
pregnancy (II-III trimester);
Should not be combined with antitussives that impede the removal of mucus.
There have been reports of severe skin lesions, such as Stevens-Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis (AGEP) and erythema multiforme exudative, coinciding with the administration of expectorants containing ambroxol. With symptomatic treatment, it is possible to erroneously prescribe “anti-cold” medications. If new lesions of the skin and mucous membranes appear, it is recommended to stop treatment with ambroxol and immediately seek medical help.
If you have impaired kidney function or severe liver disease, ambroxol should not be used without a doctor’s prescription. When using ambroxol, as with any drug that is metabolized in the liver and excreted by the kidneys, accumulation of ambroxol metabolites produced in the liver should be expected in patients with severe renal failure.
Children
In children under 2 years of age, the drug can only be used as prescribed by a doctor.
Driving vehicles and working with machinery
There were no cases of the drug affecting the ability to drive vehicles and machinery. Studies on the effect of the drug on the ability to drive vehicles and engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions have not been conducted.
Ambrobene contains sodium
Ambrobene contains less than 1 mmol (23 mg) sodium per ampoule, meaning it is essentially sodium-free.
Active ingredient
Active ingredient
Ambroxol
Composition
Composition
The active ingredient is ambroxol.
Each ampoule (2 ml) contains 15.0 mg ambroxol (as hydrochloride).
Other ingredients (excipients) are: citric acid monohydrate, sodium chloride, sodium hydrogen phosphate heptahydrate, hydrochloric acid 25%, water for injection.
Pregnancy
Pregnancy
If you are pregnant or breastfeeding, think you may be pregnant, or are planning a pregnancy, consult your doctor or pharmacist before using this drug.
Pregnancy
Ambroxol penetrates the placental barrier. Precautions must be taken when using ambroxol during pregnancy. The drug is contraindicated in the first trimester. In the second and third trimesters of pregnancy, the use of the drug is possible only if the potential benefit to the mother outweighs the potential risk to the fetus.
Breastfeeding
Ambroxol may be excreted in breast milk. Despite the fact that no undesirable effects were observed in breast-fed children, the use of ambroxol is contraindicated during lactation (breastfeeding).
Fertility
Data from preclinical studies have not shown a negative effect on fertility (the ability to conceive and bear offspring).
Contraindications
Contraindications
Do not use Ambrobene:
• if you are allergic to ambroxol or any of the other ingredients of this medicine (listed in section 6 of the leaflet);
• during pregnancy (first trimester);
• during lactation (breastfeeding).
If any of the following conditions apply to you, do not use Ambrobene. If you are unsure, consult your doctor or pharmacist.
Side Effects
Side Effects
Like all medicines, Ambrobene can cause side effects, although not everyone gets them.
If you notice any of the following serious adverse reactions, stop using Ambrobene and seek medical attention immediately:
allergic reactions (urticaria, skin rash, angioedema of the face, shortness of breath, itching, fever);
anaphylactic reactions, including anaphylactic shock.
Other adverse reactions
Tell your doctor if you notice any of the following side effects:
Common – may occur in no more than 1 in 10 people:
dysgeusia (loss or distortion of certain taste stimuli);
pharyngeal hypoesthesia (decreased sensitivity in the pharynx);
nausea, oral hypoesthesia (decreased sensitivity in the oral cavity).
Uncommon – may occur in no more than 1 person in 100:
vomiting, diarrhea, dyspepsia (indigestion), abdominal pain, dry mouth.
Rare – may occur in no more than 1 person in 1000:
dry throat;
rash, urticaria.
Frequency unknown – the frequency of occurrence cannot be determined based on the available data:
anaphylactic reactions, anaphylactic shock, hypersensitivity;
angioedema, itching;
severe skin reactions, including erythema multiforme, Stevens-Johnson syndrome/toxic epidermal necrolysis and acute generalized exanthematous pustulosis.
Interaction
Interaction
Tell your doctor or pharmacist if you are taking, have recently taken, or may start taking any other drugs, including:
– antitussive drugs (for example, codeine), since due to the suppression of the cough reflex there may be a danger of accumulation of sputum in the airway with difficulty in its release;
– antibiotics: amoxicillin, cefuroxime, erythromycin and doxycycline, since the concentration of the latter in sputum and bronchial secretions increases.
Overdose
Overdose
Symptoms:
There were no signs of intoxication with an overdose of ambroxol. There are reports of nervous agitation and diarrhea.
Ambroxol is well tolerated when administered parenterally at a dose of up to 15 mg/kg/day and when administered orally at a dose of up to 25 mg/kg/day.
In case of severe overdose, increased salivation, retching, vomiting, and decreased blood pressure are possible.
Treatment:
Urgent measures, such as inducing vomiting and gastric lavage, should only be used in cases of severe overdose. Symptomatic treatment is indicated.
Storage conditions
Storage conditions
Keep the drug out of the reach of children and so that the child cannot see it.
Do not use the drug after the expiration date (shelf life) indicated on the package after the words “Best before:”. The expiration date is the last day of the given month.
Store at a temperature not exceeding 25 °C.
The shelf life of the drug is 5 years.
Do not throw the drug into the sewer. Ask your pharmacist about how to properly discard medications that are no longer needed. These measures will help protect the environment.
Manufacturer
Manufacturer
Merkle GmbH, Germany
Additional information
| Shelf life | 5 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 25 °C |
| Manufacturer | Merkle GmbH, Germany |
| Medication form | solution |
| Brand | Merkle GmbH |
Other forms…
Related products
Buy Ambrobene, 15 mg/2 ml 2 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.