Subtotal: €6.92
Alfa Normix, 200 mg 12 pcs.
€25.92 €21.60
Pharmacological action Pharmacological action – antibacterial.
Pharmacodynamics
Rifaximin is a broad spectrum antibiotic of the rifamycin group. Like other representatives of this group it irreversibly binds beta-subunits of bacteria enzyme DNA-dependent RNA polymerase and, therefore, inhibits synthesis of RNA and proteins in bacteria.
As a result of irreversible binding to the enzyme, rifaximin exhibits bactericidal properties against sensitive bacteria. The drug has a wide spectrum of antimicrobial activity including most gram-negative and gram-positive, aerobic and anaerobic bacteria.
The wide antibacterial spectrum of rifaximin helps to reduce pathogenic intestinal bacterial load that causes some pathological conditions.
The drug reduces:
– production by bacteria of ammonia and other toxic compounds, which in the case of severe liver disease, accompanied by impaired detoxification process, play a role in the pathogenesis and clinical manifestations of hepatic encephalopathy;
– increased bacterial proliferation in gut microbial overgrowth syndrome;
– the presence of bacteria in the colonic diverticulum, which may cause inflammation in and around the diverticular sac and may play a key role in the development of symptoms and complications of diverticular disease;
– an antigenic stimulus that, in the presence of genetically determined defects in mucosal immunoregulation and/or protective function, may initiate or permanently maintain chronic intestinal inflammation;
The risk of developing infectious complications in colorectal surgery.
The mechanism of resistance. The development of resistance to rifaximin is due to reversible damage to the rpoB gene that encodes bacterial RNA polymerase. The incidence of resistant subpopulations among bacteria isolated from patients with traveler’s diarrhea was low.
According to clinical studies, a three-day course of rifaximin therapy in patients with traveler’s diarrhea was not accompanied by the occurrence of resistant Gram-positive (enterococci) and Gram-negative (E. coli) bacteria. When rifaximin was repeatedly administered at high doses in healthy volunteers and patients with inflammatory bowel disease, rifaximin-resistant strains appeared, but they did not colonize the GI tract or displace rifaximin-sensitive strains.
The resistant strains quickly disappeared when therapy was discontinued. Experimental and clinical data suggest that the use of rifaximin in patients with traveler’s diarrhea and latent Mycobacterium tuberculosis and Neisseria meningitidis infection will not be accompanied by selection of rifampicin-resistant strains.
Sensitivity. In vitro sensitivity testing cannot be used to determine the sensitivity or resistance of bacteria to rifaximin. There is currently insufficient clinical data to establish limits for evaluating sensitivity tests. Rifaximin was evaluated in vitro against traveler’s diarrhea pathogens from four regions of the world: enterotoxigenic and enteroaggregative strains of E. coli, Salmonella spp., Shigella spp., noncholera vibrios, Plesiomonas spp., Aeromonas spp. and Campylobacter spp. The MPC90 for the isolated strains was 32 μg/mL, and this level was easily attainable in the intestinal lumen as a result of the high concentration of rifaximin in the feces. Because rifaximin in polymorphic alpha has low gastrointestinal absorption and acts locally in the gut lumen, it may not be clinically effective against invasive bacteria even if these bacteria are sensitive to it in vitro.
Pharmacokinetics
Intake. Rifaximin in polymorphic form alpha is practically not absorbed when administered orally (less than 1%). When repeated administration in healthy volunteers and patients with damaged intestinal mucosa and inflammatory bowel disease, plasma concentrations are very low (less than 10 ng/ml). When using the drug 30 min after a fatty meal, an increase in systemic absorption of rifaximin of no clinical significance was observed.
Distribution. Rifaximin is moderately bound to plasma proteins. Protein binding in healthy volunteers is 67.5%, in patients with hepatic impairment – 62%.
Elimation. It is excreted unchanged in intestinal form (96.9% of the administered dose), because it is not degraded and metabolized during passage through the gastrointestinal tract.
Rifaximin detected with labeled isotopes in urine is not more than 0.025% of the oral dose. Less than 0.01% of the dose is excreted by the kidneys as 25-desacetylrifaximin, the only rifaximin metabolite identified in humans. Renal excretion of 14C rifaximin does not exceed 0.4%. Systemic exposure is nonlinear, dose-dependent, which is comparable to the absorption of rifaximin, possibly limited by the rate of dissolution.
Particular patient groups
With renal impairment. There are no clinical data on the use of rifaximin in renal failure.
With hepatic impairment. Systemic exposure in patients with hepatic impairment is greater than in healthy volunteers. Increased systemic exposure in these patients should be considered in light of the local action of rifaximin in the gut and its low systemic bioavailability, as well as available data on the safety of rifaximin in patients with cirrhosis.
Children. The pharmacokinetics of rifaximin in children have not been studied.
Indications
treatment of gastrointestinal infections caused by bacteria sensitive to rifaximin, namely:
– acute gastrointestinal infections;
– traveler’s diarrhea;
– syndrome of excessive growth of microorganisms in the intestines;
– hepatic encephalopathy;
– symptomatic uncomplicated diverticular disease of the colon;
– chronic intestinal inflammation.
prevention of infectious complications during colorectal surgery.
Pharmacological effect
Pharmacological action
Pharmacological action – antibacterial.
Pharmacodynamics
Rifaximin is a broad-spectrum antibiotic from the rifamycin group. Like other representatives of this group, it irreversibly binds the beta subunits of the bacterial enzyme DNA-dependent RNA polymerase and, therefore, inhibits the synthesis of RNA and bacterial proteins.
As a result of irreversible binding to the enzyme, rifaximin exhibits bactericidal properties against sensitive bacteria. The drug has a wide spectrum of antimicrobial activity, including most gram-negative and gram-positive, aerobic and anaerobic bacteria.
The broad antibacterial spectrum of rifaximin helps reduce the pathogenic intestinal bacterial load, which causes some pathological conditions.
The drug reduces:
– the formation by bacteria of ammonia and other toxic compounds, which in the case of severe liver disease, accompanied by a violation of the detoxification process, play a role in the pathogenesis and clinical manifestations of hepatic encephalopathy;
– increased proliferation of bacteria in the syndrome of excessive growth of microorganisms in the intestines;
– the presence of bacteria in the colon diverticulum, which can cause inflammation in and around the diverticular sac and may play a key role in the development of symptoms and complications of diverticular disease;
– an antigenic stimulus that, in the presence of genetically determined defects in mucosal immunoregulation and/or protective function, can initiate or constantly maintain chronic intestinal inflammation;
– the risk of developing infectious complications during colorectal surgery.
Mechanism of resistance. The development of resistance to rifaximin is caused by reversible damage to the rpoB gene, which encodes bacterial RNA polymerase. The occurrence of resistant subpopulations among bacteria isolated from patients with traveler’s diarrhea was low.
According to clinical studies, a three-day course of rifaximin therapy in patients with traveler’s diarrhea was not accompanied by the emergence of resistant gram-positive (enterococci) and gram-negative (Escherichia coli) bacteria. With repeated use of rifaximin in high doses in healthy volunteers and patients with inflammatory bowel diseases, rifaximin-resistant strains appeared, but they did not colonize the gastrointestinal tract and did not displace rifax-resistant strains.
When therapy was stopped, resistant strains quickly disappeared. Experimental and clinical data suggest that the use of rifaximin in patients with traveler’s diarrhea and latent infection with Mycobacterium tuberculosis and Neisseria meningitidis will not be associated with the selection of rifampicin-resistant strains.
Sensitivity. In vitro susceptibility testing cannot be used to determine the sensitivity or resistance of bacteria to rifaximin. At present, clinical data are insufficient to establish cutoff values for evaluating susceptibility tests. Rifaximin was evaluated in vitro against pathogens of travelers’ diarrhea from four regions of the world: enterotoxigenic and enteroaggregative strains of E. coli, Salmonella spp., Shigella spp., non-cholera Vibrio, Plesiomonas spp., Aeromonas spp. and Campylobacter spp. The MIC90 for the isolated strains was 32 μg/ml, a level easily achievable in the intestinal lumen as a result of the high concentration of rifaximin in feces. Because rifaximin alpha polymorph has low absorption from the gastrointestinal tract and acts locally in the intestinal lumen, it may not be clinically effective against invasive bacteria, even if these bacteria are sensitive to it in vitro.
Pharmacokinetics
Suction. Rifaximin in the alpha polymorphic form is practically not absorbed when taken orally (less than 1%). With repeated use in healthy volunteers and patients with damaged intestinal mucosa, in inflammatory bowel diseases, plasma concentrations are very low (less than 10 ng/ml). When using the drug 30 minutes after eating a fatty meal, an increase in the systemic absorption of rifaximin, which was not clinically significant, was noted.
Distribution. Rifaximin is moderately bound to plasma proteins. The binding to proteins in healthy volunteers is 67.5%, in patients with liver failure – 62%.
Excretion. It is excreted unchanged from the body by the intestines (96.9% of the dose taken), because does not undergo degradation or metabolism when passing through the gastrointestinal tract.
Rifaximin detected using labeled isotopes in urine is no more than 0.025% of the dose taken orally. Less than 0.01% of the dose is excreted by the kidneys as 25-desacetylrifaximin, the only metabolite of rifaximin identified in humans. Renal excretion of 14C rifaximin does not exceed 0.4%. Systemic exposure is nonlinear and dose-dependent, which is comparable to the absorption of rifaximin, possibly limited by the rate of dissolution.
Special patient groups
With kidney failure. There are no clinical data on the use of rifaximin in renal failure.
With liver failure. Systemic exposure in patients with liver failure exceeds that in healthy volunteers. The increased systemic exposure in these patients should be considered in light of the local action of rifaximin in the intestine and its low systemic bioavailability, as well as the available data on the safety of rifaximin in patients with cirrhosis.
Children. The pharmacokinetics of rifaximin in children has not been studied.
Special instructions
Clinical data indicate that Alpha Normix® is ineffective in the treatment of intestinal infections caused by Campylobacter jejuni, Salmonella spp., Shigella spp., which cause frequent diarrhea, fever, and bloody stool. Alpha Normix® is not recommended for use if patients have fever and loose, bloody stools. Alpha Normix® should be discontinued if symptoms of diarrhea worsen or persist for more than 48 hours. Other antibacterial therapy should be prescribed. Treatment of traveler’s diarrhea should not exceed 3 days.
It is known that Clostridium difficile-associated diarrhea can develop with the use of almost all antibacterial agents, including the drug Alpha Normix®. A potential relationship between Alpha Normix® and the development of Clostridium difficile-associated diarrhea and pseudomembranous colitis cannot be excluded. There is no experience with the use of rifaximin in combination with other rifamycins.
Caution should be exercised when taking rifaximin concomitantly with a P-gp inhibitor such as cyclosporine. Patients should be warned that, despite the slight absorption of rifaximin (less than 1%), it can cause urine to turn reddish in color: this is due to the active substance rifaximin, which, like most antibiotics of this series (rifamycins), has a reddish-orange color.
If a superinfection caused by microorganisms that are insensitive to rifaximin develops, taking Alpha Normix® should be stopped and appropriate therapy should be prescribed.
Due to the effect of Alpha Normix® on the intestinal flora, the effectiveness of oral contraceptives containing estrogens may decrease after taking it. It is recommended to use additional contraceptive measures when taking Alpha Normix®, especially if the estrogen content in oral contraceptives is less than 50 mcg.
Taking Alpha Normix® is possible no earlier than 2 hours after taking activated carbon.
Influence on the ability to drive vehicles and machinery. Although dizziness and drowsiness are observed when using the drug Alpha Normix®, it does not have a significant effect on the ability to drive vehicles and engage in activities that require increased attention and speed of psychomotor reactions. If dizziness and drowsiness occur while using the drug, you should refrain from performing these activities.
Active ingredient
Rifaximin
Composition
Each film-coated tablet contains:
Active substance:
rifaximin with polymorphic structure alpha 200 mg.
Excipients:
sodium carboxymethyl starch 15 mg,
glyceryl palmitostearate 18 mg,
colloidal silicon dioxide 1 mg,
talc 1 mg,
microcrystalline cellulose 115 mg.
Film casing:
hypromellose 5.15 mg,
titanium dioxide (E171) 1.5 mg,
disodium edetate 0.02 mg,
propylene glycol 0.5 mg,
iron oxide red (E172) 0.15 mg.
Pregnancy
Data on the use of Alpha Normix® during pregnancy are very limited.
Animal studies have shown a transient effect of rifaximin on ossification and skeletal structure in the fetus. The clinical significance of these results is unknown. The use of Alpha Normix® during pregnancy is not recommended.
It is not known whether rifaximin passes into breast milk. A risk to a breastfed baby cannot be ruled out. To decide whether to continue taking rifaximin during breastfeeding, it is necessary to assess the balance of risk for the child and benefit for the mother.
Contraindications
– hypersensitivity to rifaximin or other rifamycins or any of the components included in the drug;
– diarrhea accompanied by fever and loose, bloody stools;
– intestinal obstruction (including partial);
– severe ulcerative lesions of the intestines;
– children under 12 years of age (efficacy and safety have not been established).
With caution: renal failure, concomitant use with oral contraceptives, concomitant use with a P-glycoprotein inhibitor such as cyclosporine.
Side Effects
Side effects are classified according to frequency as follows: very common (≥1/10), common (≥1/100 – ˂1/10), uncommon (≥1/1000 – ˂1/100), rare (≥1/10000 – ˂1/1000), very rare (˂1/10000), unknown (frequency cannot be determined based on available data). data).
From the cardiovascular system:
Uncommon: palpitations, flushing of blood to the skin of the face, increased blood pressure.
From the blood side:
Uncommon: lymphocytosis, monocytosis, neutropenia.
Unknown: thrombocytopenia.
From the immune system:
Not known: anaphylactic reactions, hypersensitivity, anaphylactic shock, laryngeal edema.
Metabolic disorders:
Uncommon: decreased appetite, dehydration.
Mental disorders:
Uncommon: pathological dreams, depressed mood, insomnia, nervousness.
From the central nervous system:
Common: dizziness, headache.
Uncommon: hypoesthesia, migraine, paresthesia, drowsiness, headache in the sinuses.
Unknown: lightheadedness, agitation.
From the side of the organ of vision:
Uncommon: diplopia.
From the inner ear:
Uncommon: ear pain, systemic dizziness.
From the respiratory system:
Uncommon: shortness of breath, dry throat, nasal congestion, pain in the oropharynx, cough, rhinorrhea.
From the gastrointestinal tract and liver:
Common: bloating, abdominal pain, constipation, diarrhea, flatulence, nausea, tenesmus, vomiting, urge to defecate.
Uncommon: pain in the upper abdomen, ascites, dyspepsia, impaired gastrointestinal motility, mucus and blood in the stool, dry lips, “hard” stools, increased aspartate aminotransferase activity, ageusia.
Not known: abnormal liver function tests, heartburn.
From the urinary system:
Uncommon: glycosuria, polyuria, pollakiuria, hematuria, proteinuria.
For the skin and subcutaneous fat:
Uncommon: rash, sunburn.
Not known: angioedema, allergic dermatitis, exfoliative dermatitis, eczema, erythema, pruritus, purpura, urticaria, erythematous rash, erythema of the palms, genital itching.
From the musculoskeletal system:
Uncommon: back pain, muscle spasm, muscle weakness, myalgia, neck pain.
Infections:
Uncommon: candidiasis, herpes simplex, nasopharyngitis, pharyngitis, infections
upper respiratory tract.
Unknown: clostridial infection.
From the reproductive system:
Uncommon: polymenorrhea.
General symptoms:
Common: fever.
Uncommon: asthenia, pain and discomfort of uncertain localization, chills, cold sweat, flu-like symptoms, peripheral edema, hyperhidrosis, facial swelling, fatigue.
Laboratory research: changes in the international normalized ratio.
Interaction
In vitro studies show that rifaximin does not inhibit cytochrome P450 isoenzymes (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, 2E1 and 3A4) and does not induce CYP1A2 and CYP2B6, but is a weak inducer of CYP3A4.
Clinical drug interaction studies indicate that in healthy volunteers, rifaximin does not have a significant effect on the pharmacokinetics of drugs metabolized by CYP3A4. In patients with impaired liver function, it cannot be excluded that rifaximin may reduce the exposure of drugs to CYP3A4 substrates (for example, warfarin, antiarrhythmics, anticonvulsants) when used simultaneously with them, because in liver failure has a higher systemic exposure compared to healthy volunteers.
In patients continuing to take warfarin and rifaximin, decreases and increases in INR (in some cases with bleeding episodes) were recorded. If coadministration of drugs is necessary, careful monitoring of the INR should be performed at the beginning and at the end of treatment. To maintain the desired level of anticoagulation, it may be necessary to adjust the dose of oral anticoagulants.
In vitro studies suggest that rifaximin is a moderate P-gp substrate and is metabolized by CYP3A4.
It is not known whether drugs that inhibit CYP3A4 increase the systemic exposure of rifaximin when used concomitantly. In healthy volunteers, coadministration of a single dose of cyclosporine (600 mg), a potent P-gp inhibitor, with a single dose of rifaximin (550 mg) resulted in 83- and 124-fold increases in mean rifaximin Cmax and AUC∞. The clinical significance of this increase for systemic exposure is unknown. Potential interactions of rifaximin with other drugs that are cleared from the cell by P-gp or other transport proteins (MRP2, MRP4, BCRP, BSEP) are unlikely.
Overdose
In clinical studies, doses of rifaximin up to 1800 mg/day were well tolerated in patients with traveler’s diarrhea. Even in patients with normal intestinal bacterial flora, rifaximin at doses up to 2400 mg/day for 7 days did not cause adverse symptoms.
In case of accidental overdose, symptomatic and supportive therapy is indicated.
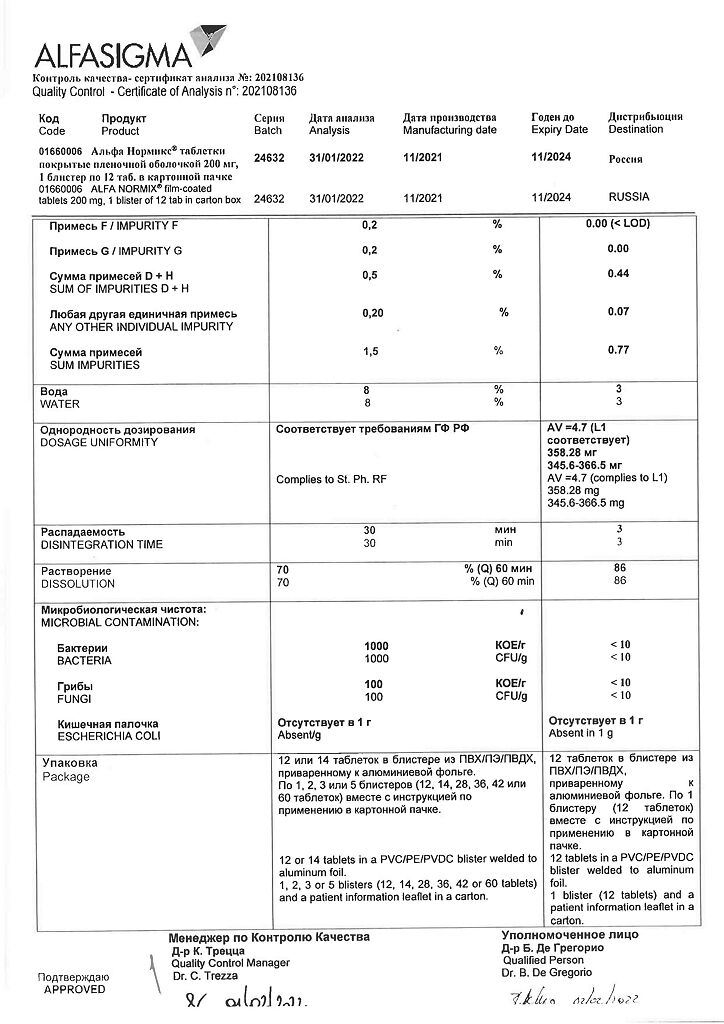
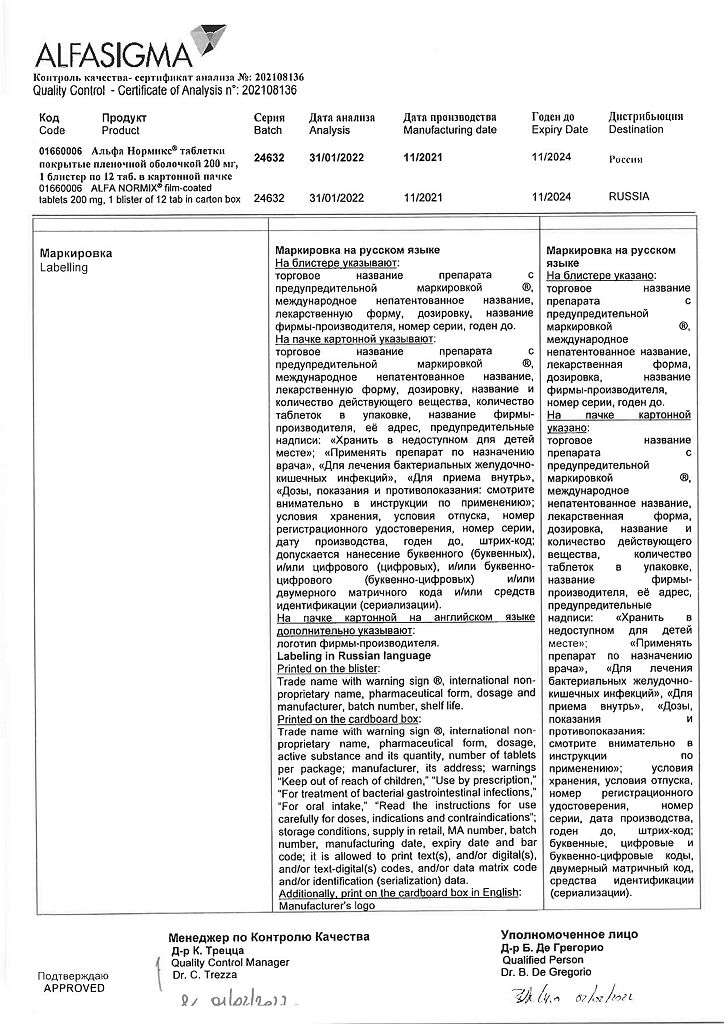
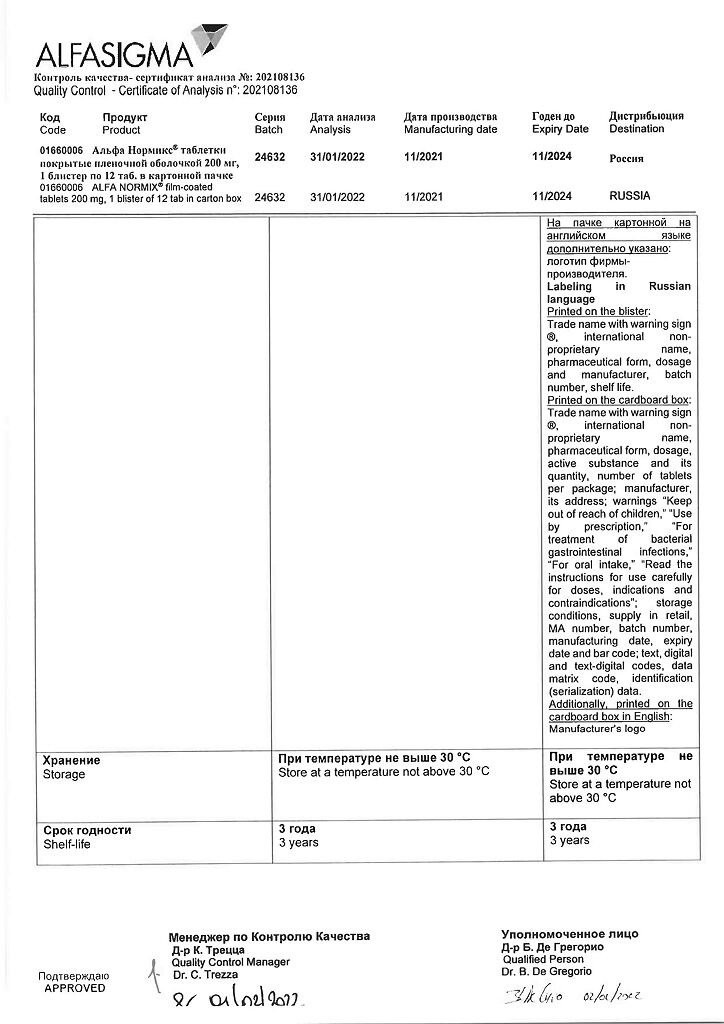
Storage conditions
At a temperature not exceeding 30 °C
Shelf life
3 years
Manufacturer
Alfasigma S.p.A., Italy
| Shelf life | 3 years |
|---|---|
| Conditions of storage | At a temperature not exceeding 30 °C |
| Manufacturer | Alphasigma S.p.A., Italy |
| Medication form | pills |
| Brand | Alphasigma S.p.A. |
Other forms…
Related products
Buy Alfa Normix, 200 mg 12 pcs. with delivery to USA, UK, Europe and over 120 other countries.