No products in the cart.
Adrenaline, 0.1% 1 ml 5 pcs
€4.33 €3.96
Description
Pharmacotherapeutic group: α- and β-adrenomimetic.
ATX code: C01СА24
Pharmacological properties
.Pharmacodynamics
Sympathomimetic acting on α- and β-adrenoreceptors. Its action is caused by activation of receptor-dependent adenylate cyclase on the inner surface of the cell membrane, increase of intracellular concentration of cyclic adenosine monophosphate (cAMP) and calcium ions (Ca2+).
In very low doses, at a rate of administration of less than 0.01 µg/kg/min may decrease blood pressure due to dilation of skeletal musculature vessels. At an administration rate of 0.04-0.1 µg/kg/min, it increases heart rate and force, blood stroke volume and minute blood volume, and reduces total peripheral vascular resistance; above 0.02 µg/kg/min, it narrows vessels, increases blood pressure (mainly systolic) and total peripheral vascular resistance. The pressor effect may cause a transient reflex slowing of the heart rate.
The relaxation of bronchial smooth muscles. Doses above 0.3 µg/kg/min decrease renal blood flow, blood flow to internal organs, and gastrointestinal tone and motility.
Dilates the pupils and helps reduce intraocular fluid production and intraocular pressure. Causes hyperglycemia (increases glycogenolysis and gluconeogenesis) and increases plasma free fatty acid content.
Increases myocardial conduction, excitability and automatism. Increases myocardial oxygen demand.
Inhibits induced release of histamine and leukotrienes by antigens, eliminates spasm of bronchioles and prevents development of edema of their mucous membranes.
Acting on α-adrenoreceptors located in the skin, mucous membranes and internal organs it causes vasoconstriction, decrease of absorption rate of local anesthetic agents, increase of duration and decrease of toxic effect of local anesthetic.
Stimulation of β2-adrenoreceptors is accompanied by increased excretion of potassium ions (K+) from the cell and may lead to hypokalemia.
In intracavernous administration, it reduces the blood filling of the cavernous bodies.
The therapeutic effect develops almost immediately with intravenous injection (duration of action 1-2 minutes), 5-10 minutes after subcutaneous injection (maximum effect after 20 minutes), with intramuscular injection the time of onset of effect is variable.
Pharmacokinetics
absorption
In intramuscular or subcutaneous administration, it is well absorbed. When administered parenterally, it breaks down quickly. It is also absorbed by endotracheal and conjunctival administration. Time to reach maximum concentration in blood when administered subcutaneously and intramuscularly is 3-10 minutes. It penetrates through the placenta and into the breast milk; it does not penetrate through the blood-brain barrier.
Metabolism
Metabolized mainly by monoaminoxidase and catechol-O-methyltransferase in sympathetic nerve endings and other tissues, as well as in the liver to form inactive metabolites. The half-life with intravenous administration is 1-2 minutes.
Evacuation
Evacuated by the kidneys mainly as metabolites: vanillylmindalic acid, sulfates, glucuronides, and in small amounts unchanged.
Indications
Indications
immediate allergic reactions (including urticaria, angioedema, anaphylactic shock) that develop when using drugs, serums, blood transfusions, eating foods, insect bites or the introduction of other allergens; exercise asthma;
bronchial asthma (relief of status asthmaticus), bronchospasm during anesthesia;
asystole (including against the background of acutely developed atrioventricular block of the third degree);
bleeding from superficial vessels of the skin and mucous membranes (including from the gums);
arterial hypotension unresponsive to adequate volumes of replacement fluids (including shock, bacteremia, open heart surgery, renal failure);
the need to prolong the action of local anesthetics;
episodes of complete atrioventricular block (with the development of syncope (Morgagni-Adams-Stokes syndrome));
stopping bleeding (as a vasoconstrictor).
Pharmacological effect
Pharmacological effect
Pharmacotherapeutic group: α- and β-adrenomimetic.
ATX code: C01CA24
Pharmacological properties
Pharmacodynamics
Sympathomimetic acting on α- and β-adrenergic receptors. The action is due to the activation of receptor-dependent adenylate cyclase on the inner surface of the cell membrane, an increase in the intracellular concentration of cyclic adenosine monophosphate (cAMP) and calcium ions (Ca2+).
In very low doses, at a rate of administration less than 0.01 mcg/kg/min, it can reduce blood pressure due to vasodilation of skeletal muscles. At an injection rate of 0.04-0.1 mcg/kg/min, it increases the frequency and strength of heart contractions, stroke volume and minute blood volume, and reduces total peripheral vascular resistance; above 0.02 mcg/kg/min constricts blood vessels, increases blood pressure (mainly systolic) and total peripheral vascular resistance. The pressor effect can cause a short-term reflex slowing of the heart rate.
Relaxes the smooth muscles of the bronchi. Doses above 0.3 mcg/kg/min reduce renal blood flow, blood supply to internal organs, tone and motility of the gastrointestinal tract.
Dilates the pupils, helps reduce the production of intraocular fluid and intraocular pressure. Causes hyperglycemia (increases glycogenolysis and gluconeogenesis) and increases plasma levels of free fatty acids.
Increases conductivity, excitability and automatism of the myocardium. Increases myocardial oxygen demand.
Inhibits the release of histamine and leukotrienes induced by antigens, eliminates spasm of bronchioles, and prevents the development of edema of their mucous membrane.
Acting on α-adrenergic receptors located in the skin, mucous membranes and internal organs, it causes vasoconstriction, reduces the rate of absorption of local anesthetics, increases the duration and reduces the toxic effect of local anesthesia.
Stimulation of β2-adrenergic receptors is accompanied by increased excretion of potassium ions (K+) from the cell and can lead to hypokalemia.
When administered intracavernosally, it reduces the blood supply to the cavernous bodies.
The therapeutic effect develops almost instantly with intravenous administration (duration of action – 1-2 minutes), 5-10 minutes after subcutaneous administration (maximum effect – after 20 minutes), with intramuscular administration – the onset of the effect is variable.
Pharmacokinetics
Suction
When administered intramuscularly or subcutaneously, it is well absorbed. When administered parenterally, it is quickly destroyed. It is also absorbed after endotracheal and conjunctival administration. The time to reach maximum concentration in the blood with subcutaneous and intramuscular administration is 3-10 minutes. Penetrates through the placenta, into breast milk, does not penetrate the blood-brain barrier.
Metabolism
Metabolized mainly by monoamine oxidase and catechol-O-methyltransferase in the endings of sympathetic nerves and other tissues, as well as in the liver with the formation of inactive metabolites. The half-life for intravenous administration is 1-2 minutes.
Removal
It is excreted by the kidneys mainly in the form of metabolites: vanillylmandelic acid, sulfates, glucuronides, and also in small quantities – unchanged.
Special instructions
Special instructions
During the treatment period, it is recommended to determine the concentration of potassium ions (K+) in the blood serum, measure blood pressure, diuresis, minute volume of blood circulation, electrocardiogram, central venous pressure, pressure in the pulmonary artery and wedge pressure in the pulmonary capillaries.
Excessive doses of epinephrine during myocardial infarction may increase ischemia by increasing myocardial oxygen demand.
Increases plasma glucose levels, which is why diabetes mellitus requires higher doses of insulin and sulfonylurea derivatives.
Epinephrine is not advisable for long-term use (constriction of peripheral blood vessels, leading to the possible development of necrosis or gangrene).
Use for correction of low blood pressure during labor is not recommended as it may delay the second stage of labor; when administered in large doses to weaken uterine contractions, it can cause prolonged uterine atony with bleeding.
When stopping treatment, doses should be reduced gradually, since sudden discontinuation of therapy can lead to severe arterial hypotension.
Easily destroyed by alkalis and oxidizing agents. Sodium metabisulfite, which is part of the drug, may cause an allergic reaction, including symptoms of anaphylaxis and bronchospasm, especially in patients with a history of asthma or allergies. Epinephrine should be used with caution in patients with tetraplegia due to the increased sensitivity of these individuals to epinephrine.
A sharp increase in blood pressure when using adrenaline can lead to the development of hemorrhage, especially in elderly patients with cardiovascular diseases.
Patients with Parkinson’s disease may experience psychomotor agitation or temporary worsening of symptoms of the disease when using epinephrine, and therefore caution should be exercised when using epinephrine in this category of individuals.
Do not re-inject into the same areas to avoid the development of tissue necrosis. Injection of the drug into the gluteal muscles is not recommended.
Epinephrine should be used with caution in elderly patients, patients with coronary heart disease, arterial hypertension, diabetes mellitus, hyperthyroidism, prostatic hyperplasia and urinary disorders.
Particular care must be taken when using epinephrine in patients with long-standing bronchial asthma, emphysema, and organic heart disease (cor pulmonale).
Epinephrine may cause cardiac arrhythmias and myocardial ischemia, especially in patients with coronary artery disease or cardiomyopathy.
Epinephrine increases cardiac output and causes peripheral vasoconstriction, which can lead to pulmonary edema.
Epinephrine causes constriction of the renal arterioles, which can lead to oliguria and renal failure.
Intramuscular injection of epinephrine should be carried out in the anterolateral surface of the thigh (in the vastus lateralis muscle). Injection of the drug into smaller muscles (for example, the deltoid muscle) is not recommended.
Rare cases of severe skin and soft tissue infections, including Clostridia necrotizing fasciitis and muscle necrosis (gas gangrene), have been reported at the site of epinephrine administration for the treatment of anaphylaxis. Patients should be warned to seek immediate medical attention if symptoms of infection such as persistent redness, warmth, swelling, or tenderness at the epinephrine injection site occur.
The drug should not be injected into the vessels of the fingers, hands and feet, because Epinephrine is a powerful vasoconstrictor and can lead to disruption of blood supply and tissue necrosis (gangrene).
Do not use the drug if color changes or sediment appears in the solution. The unused portion of the solution should be discarded.
Impact on the ability to drive vehicles and machinery
After using the drug, the doctor must individually, in each specific case, decide on the patient’s permission to drive vehicles or engage in other potentially hazardous activities that require increased concentration and speed of psychomotor reactions.
Active ingredient
Active ingredient
Epinephrine
Composition
Composition
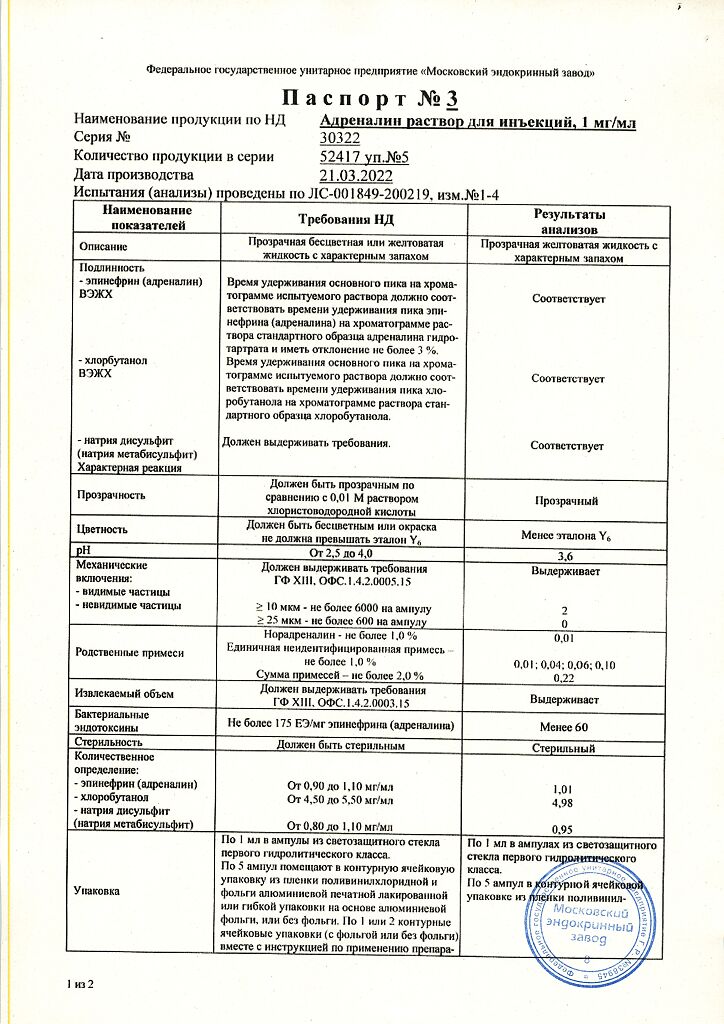
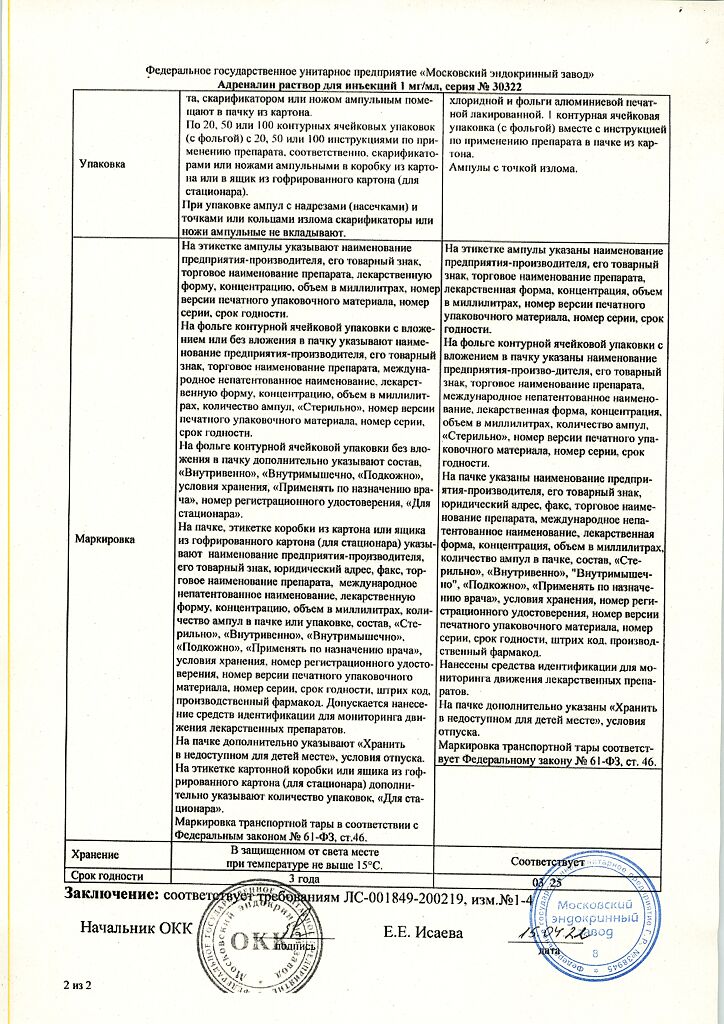
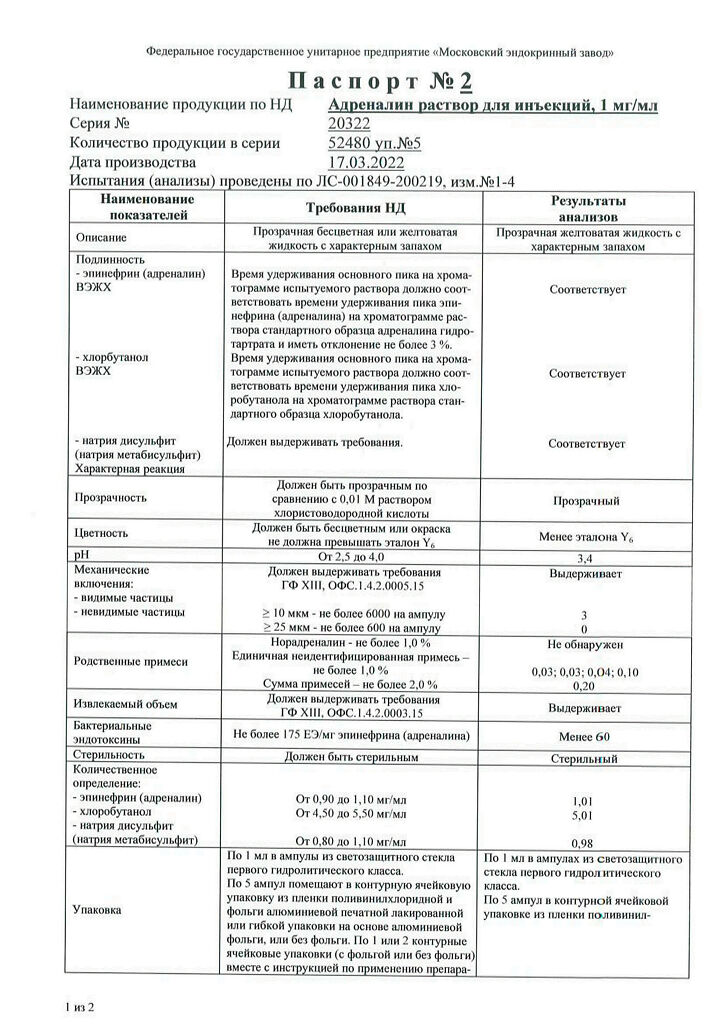
1 ml of solution contains:
Active ingredient:
epinephrine (adrenaline) – 1.00 mg
Excipients:
sodium chloride – 8.00 mg, sodium disulfite (sodium metabisulfite) – 1.00 mg, chlorobutanol hemihydrate (chlorobutanol hydrate), equivalent to 5.00 mg of chlorobutanol, disodium edetate – 0.50 mg, glycerol (glycerol) – 60.00 mg, hydrochloric acid (hydrochloric acid) – to pH 2.5-4.0, water for injection – up to 1 ml.
Pregnancy
Pregnancy
There are no strictly controlled studies of the use of epinephrine in pregnant women. Epinephrine crosses the placenta. A statistically consistent relationship has been established between the occurrence of malformations and inguinal hernia in children when using adrenaline in pregnant women, especially in the first trimester or throughout pregnancy. There is a report of a single case of anoxia in the fetus (with intravenous administration of epinephrine). Injection of epinephrine can cause tachycardia, heart rhythm disturbances, including additional systolic beats, etc. in the fetus. Epinephrine should not be used in pregnant women with blood pressure above 130/80 mmHg. Art.
Animal experiments have shown that when administered in doses 25 times higher than the recommended dose for humans, epinephrine causes a teratogenic effect.
Epinephrine should be used during pregnancy only if the potential benefit to the mother outweighs the possible risk to the fetus. Use for correction of hypotension during labor is not recommended as it may delay the second stage of labor; when administered in large doses to weaken uterine contractions, it can cause prolonged uterine atony with bleeding. Epinephrine should not be used during childbirth; use is only possible if it is prescribed for health reasons.
If treatment with epinephrine is necessary during breastfeeding, breastfeeding should be discontinued.
Contraindications
Contraindications
hypersensitivity to any of the components of the drug;
hypertrophic obstructive cardiomyopathy, tachyarrhythmia, ventricular fibrillation, chronic heart failure stage II B – III;
pheochromocytoma;
acute and chronic arterial insufficiency;
hyperkalemia;
shock of non-allergic origin (including cardiogenic, traumatic, hemorrhagic);
cold injury;
organic brain damage;
angle-closure glaucoma;
children under 18 years of age (except for conditions directly threatening life);
pregnancy, breastfeeding period;
simultaneous use of inhalation agents for general anesthesia (halothane).
Epinephrine in combination with local anesthetics is not used for local anesthesia of fingers and toes due to the risk of ischemic tissue damage.
In emergency conditions, all contraindications are relative.
With caution
metabolic acidosis;
coronary heart disease;
arterial hypertension;
hypercapnia, hypoxia;
atrial fibrillation;
ventricular arrhythmia;
pulmonary hypertension;
hypovolemia;
myocardial infarction;
occlusive vascular diseases (including in the history – arterial embolism, atherosclerosis, Buerger’s disease, diabetic endarteritis, Raynaud’s disease);
diabetes mellitus;
hyperthyroidism;
long-standing bronchial asthma and emphysema;
cerebral atherosclerosis;
Parkinson’s disease;
tetraplegia;
convulsive syndrome;
prostatic hyperplasia and/or difficulty urinating;
old age;
paresis and paralysis;
increased tendon reflexes in spinal cord injury.
Side Effects
Side Effects
The incidence of side effects was determined according to the World Health Organization classification: very common (≥ 1/10), common (≥ 1/100, < 1/10), uncommon (≥ 1/1000, < 1/100), rare (≥ 1/10000, < 1/1000), very rare (< 1/10000), including individual reports.
Immune system disorders: uncommon – angioedema, bronchospasm, skin rash, erythema multiforme.
Nervous system disorders: often – headache, anxiety, tremor, tic; uncommon – dizziness, nervousness, fatigue, nausea, vomiting, personality disorders (psychomotor agitation, disorientation, memory impairment, psychotic disorders: aggressive or panicky behavior, schizophrenia-like disorders, paranoia), sleep disturbance, muscle twitching.
Cardiac disorders: uncommon – angina pectoris, bradycardia or tachycardia, palpitations, increased or decreased blood pressure, at high doses – ventricular arrhythmias (including ventricular fibrillation); rarely – arrhythmia, chest pain, pulmonary edema.
Vascular disorders: uncommon – increased or decreased blood pressure; rarely – pulmonary edema.
Gastrointestinal disorders: often – nausea, vomiting.
Skin and subcutaneous tissue disorders: uncommon – increased sweating.
Disorders of the kidneys and urinary tract: rarely – difficult and painful urination (with prostatic hyperplasia).
General disorders and disorders at the injection site: uncommon – pain or burning at the site of intramuscular injection.
Laboratory and instrumental data: rarely – hypokalemia.
Interaction
Interaction
Epinephrine antagonists are α- and β-adrenergic receptor blockers.
The effectiveness of epinephrine is reduced in patients with severe anaphylactic reactions taking beta-blockers. In this case, salbutamol is used intravenously.
Use together with other adrenergic agonists may enhance the effect of epinephrine.
Reduces the effects of narcotic analgesics and sleeping pills.
When used simultaneously with cardiac glycosides, quinidine, tricyclic antidepressants, dopamine, inhalation anesthetics (enflurane, halothane, isoflurane, methoxyflurane), cocaine, the risk of developing arrhythmias increases (they should be used together with extreme caution or not at all); with other adrenergic agonists – increased severity of side effects from the cardiovascular system; with antihypertensive drugs – a decrease in their effectiveness.
With diuretics, the pressor effect of epinephrine may be increased.
Simultaneous use with drugs that inhibit monoamine oxidase (procarbazine, selegiline), as well as furazolidone, can cause a sudden and pronounced increase in blood pressure, hyperpyretic crisis, headache, arrhythmia, vomiting; with nitrates – weakening of their therapeutic effect; with phenoxybenzamine – increased antihypertensive effect and tachycardia; with phenytoin – a sudden decrease in blood pressure and bradycardia (depending on the dose and rate of administration); with thyroid hormone preparations – mutual enhancement of action. With drugs that prolong the QT interval (with antiarrhythmic drugs (such as lidocaine, amiodarone, sotalol, etc.), with antibiotics (such as erythromycin, levofloxacin, etc.), with antihistamines (such as loratadine, diphenhydramine, etc.), with tricyclic and tetracyclic antidepressants (such as amitriptyline, imipramine, sertraline, chlorpromazine, etc.), with antipsychotics (such as haloperidol, risperidone, etc.), with dopamine receptor antagonists (such as domperidone, etc.), with antimalarial drugs (such as chloroquine, mefloquine, etc.), with antifungal drugs (such as ketoconazole, fluconazole, etc.), with antihypertensive drugs (such as indapamide, ephedrine, etc.)) – may cause a prolongation of the QT interval.
Simultaneous use with diatriazotes, iothalamic or ioxaglic acids – increased neurological effects, with ergot alkaloids – increased vasoconstrictor effect (up to severe ischemia and the development of gangrene).
Reduces the effect of insulin and other hypoglycemic drugs.
Overdose
Overdose
Symptoms: excessive increase in blood pressure, tachycardia alternating with bradycardia, rhythm disturbances (including atrial and ventricular fibrillation), coldness and pallor of the skin, vomiting, headache, metabolic acidosis, myocardial infarction, cerebral hemorrhage (especially in elderly patients), pulmonary edema, death.
Treatment: stop administering the drug, symptomatic therapy – to lower blood pressure – α-blockers (phentolamine), for arrhythmia – β-blockers (propranolol).
Storage conditions
Storage conditions
In a place protected from light at a temperature not exceeding 15 ºС.
Keep out of the reach of children.
Shelf life
Shelf life
3 years. Do not use after the expiration date stated on the package.
Manufacturer
Manufacturer
Moscow Endocrine Plant, Russia
Additional information
| Shelf life | 3 years. Do not use after the expiration date stated on the package. |
|---|---|
| Conditions of storage | In the dark place at a temperature no higher than 15 ºС. Keep out of reach of children. |
| Manufacturer | Moscow Endocrine Plant, Russia |
| Medication form | solution for injection |
| Brand | Moscow Endocrine Plant |
Related products
Buy Adrenaline, 0.1% 1 ml 5 pcs with delivery to USA, UK, Europe and over 120 other countries.