No products in the cart.
Actrapid® NM, 100 me/ml 10 ml
€11.44 €10.14
Description
Actrapid NM is a short-acting insulin preparation. Interacting with the specific receptor of the outer membrane of cells, it forms an insulin-receptor complex.
By increasing the synthesis of cAMP (in fat cells and liver cells) or directly penetrating into the cell (muscles) the insulin-receptor complex stimulates intracellular processes, including the synthesis of several key enzymes (hexokinase, pyruvate kinase, glycogen synthetase and others).
The reduction of glucose concentration in blood is conditioned by the increase of its intracellular transport, increased absorption and assimilation by tissues, stimulation of lipogenesis, glycogenogenesis and protein synthesis, reduction of glucose production by liver (decrease of glycogen decay) and others.
After injection the action comes within 20-30 minutes, reaches the maximum within 1-3 hours and lasts for 5-8 hours, depending on the dose. The duration of action of the drug depends on the dose, method, place of administration and has significant individual characteristics.
Indications
Indications
Insulin-dependent diabetes mellitus
Pharmacological effect
Pharmacological effect
Actrapid NM is a short-acting insulin preparation. Interacting with a specific receptor on the outer cell membrane, it forms an insulin receptor complex.
By increasing the synthesis of cAMP (in fat cells and liver cells) or directly penetrating the cell (muscles), the insulin receptor complex stimulates intracellular processes, incl. synthesis of a number of key enzymes (hexokinase, pyruvate kinase, glycogen synthetase, etc.).
The decrease in the concentration of glucose in the blood is due to an increase in its intracellular transport, increased absorption and assimilation by tissues, stimulation of lipogenesis, glycogenogenesis, protein synthesis, a decrease in the rate of glucose production by the liver (reduced glycogen breakdown), etc.
After subcutaneous injection, the effect occurs within 20-30 minutes, reaches a maximum after 1-3 hours and lasts, depending on the dose, 5-8 hours. The duration of action of the drug depends on the dose, method, place of administration and has significant individual characteristics.
Special instructions
Special instructions
On the recommendation of a doctor, treatment with Actrapid NM Penfill can be combined with the use of long-acting insulin preparations. Patients receiving more than 100 units of insulin per day should be hospitalized when changing the drug.
When the liver is damaged, the need for insulin decreases.
With kidney damage, the need for insulin decreases.
Impact on the ability to drive vehicles and operate machinery
After transferring the patient to biosynthetic human insulin, the ability to drive a car and engage in other potentially hazardous activities that require increased attention and rapid psychomotor reactions may temporarily deteriorate.
Active ingredient
Active ingredient
Insulin soluble human genetically engineered
Composition
Composition
Active ingredient:
soluble insulin (human genetically engineered) 100 IU* * 1 IU corresponds to 35 mcg of anhydrous human insulin.
Excipients:
zinc chloride,
glycerol,
metacresol,
hydrochloric acid and/or sodium hydroxide (to maintain pH level),
water d/i.
Contraindications
Contraindications
Hypersensitivity to the components of the drug, hypoglycemia.
Side Effects
Side Effects
Adverse reactions observed in patients during therapy with Actrapid NM were predominantly dose-dependent and were due to the pharmacological action of insulin. As with other insulin medications, the most common side effect is hypoglycemia. It develops in cases where the dose of insulin significantly exceeds the need for it. During clinical studies, as well as during the use of the drug after its release on the consumer market, it was found that the frequency of hypoglycemia varies in different patient populations and when using different dosage regimens, so it is not possible to indicate exact frequency values.
Severe hypoglycemia may cause loss of consciousness and/or seizures, temporary or permanent impairment of brain function, and even death. Clinical studies have shown that the incidence of hypoglycemia was generally no different between patients receiving human insulin and patients receiving insulin aspart.
Below are the frequencies of adverse reactions identified during clinical studies that were assessed as associated with the use of the drug Actrapid NM. The frequency was determined as follows: uncommon (> 1/1000,
From the immune system: infrequently – urticaria, rash; very rarely – anaphylactic reactions. Symptoms of generalized hypersensitivity may include generalized skin rash, itching, sweating, gastrointestinal disorders, angioedema, shortness of breath, palpitations, decreased blood pressure, fainting/loss of consciousness. Generalized hypersensitivity reactions can be life-threatening.
From the nervous system: very rarely – peripheral neuropathy. If improvement in blood glucose control is achieved very quickly, a condition called “acute painful neuropathy” may develop, which is usually reversible.
From the side of the organ of vision: infrequently – refractive errors. Refractive errors are usually noted at the initial stage of insulin therapy. As a rule, these symptoms are reversible; very rarely – diabetic retinopathy. If adequate glycemic control is maintained over a long period of time, the risk of progression of diabetic retinopathy is reduced. However, intensification of insulin therapy with a sharp improvement in glycemic control may lead to a temporary increase in the severity of diabetic retinopathy.
From the skin and subcutaneous tissues: rarely – lipodystrophy. Lipodystrophy can develop at the injection site if the injection site is not constantly changed within the same area of the body.
On the part of the body as a whole, as well as reactions at the injection site: uncommon – reactions at the injection site. During insulin therapy, reactions may occur at the injection site (skin redness, swelling, itching, pain, hematoma formation at the injection site). However, in most cases, these reactions are transient and disappear as therapy continues. Swelling is usually noted at the initial stage of insulin therapy. As a rule, this symptom is transient.
Interaction
Interaction
The hypoglycemic effect of insulin is enhanced by MAO inhibitors, non-selective beta-blockers, sulfonamides, anabolic steroids, tetracyclines, clofibrate, cyclophosphamide, fenfluramine; preparations containing ethanol.
The hypoglycemic effect of insulin is reduced by oral contraceptives, glucocorticoids, thyroid hormones, thiazide diuretics, heparin, lithium preparations, tricyclic antidepressants.
Under the influence of reserpine and salicylates, it is possible to both weaken and enhance the action of insulin.
Pharmaceutical interactions
Ethanol and various disinfectants can reduce the biological activity of insulin.
Overdose
Overdose
Symptoms: initial symptoms of hypoglycemia: sudden increase in sweating, palpitations, tremor, hunger, agitation, paresthesia in the mouth, pallor, headache, sleep disturbances. In severe cases of overdose – coma.
Treatment: The patient can eliminate mild hypoglycemia himself by taking sugar or sugar-rich foods. In severe cases, 1 mg of glucagon is administered subcutaneously or intramuscularly. If necessary, therapy is continued with intravenous administration of concentrated glucose solutions.
Storage conditions
Storage conditions
Store in the refrigerator at a temperature of 2°C to 8°C (not too close to the freezer) in a cardboard box.
Shelf life
Shelf life
Shelf life: 2.5 years.
Opened bottle – 6 weeks.
Manufacturer
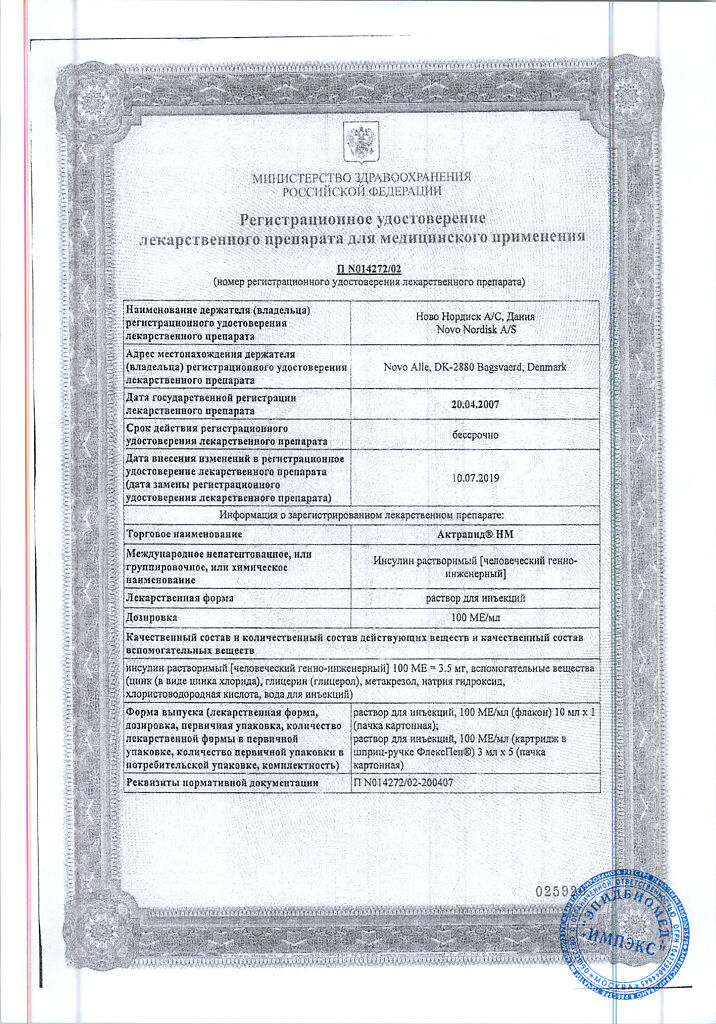
Manufacturer
Novo Nordisk A/S, Denmark
Additional information
| Shelf life | Shelf life: 2.5 years. Opened bottle – 6 weeks. |
|---|---|
| Conditions of storage | Store in the refrigerator at 2 ° C to 8 ° C (not too close to the freezer) in a cardboard package. |
| Manufacturer | Novo Nordisk A/S, Denmark |
| Medication form | solution for injection |
| Brand | Novo Nordisk A/S |
Related products
Buy Actrapid® NM, 100 me/ml 10 ml with delivery to USA, UK, Europe and over 120 other countries.