No products in the cart.
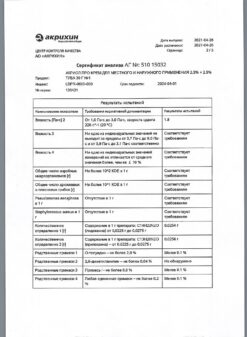
Acryol Pro, cream 2.5% + 2.5% 5 g
€8.17 €7.15
EAN: 4601969007985
SKU: 280031
Categories: Anesthesia and resuscitation, Local anesthetics, Medicine
Description
Acriol Pro is a combined preparation containing lidocaine and prilocaine, local anesthetics of the amide type. Anesthesia of the skin is caused by the penetration of lidocaine and prilocaine into the layers of the epidermis and dermis. The degree of anesthesia depends on the dose of the drug and the duration of application.
Intact skin:
After application to intact skin for 1-2 hours, the duration of anesthesia after removal of the occlusive dressing is 2 hours. No differences in efficacy (including time to anesthetic effect) and safety were found when the drug was applied to intact skin in elderly (65-96 years) and younger patients.
Transient pallor or redness of the skin may occur due to the effect of the drug on superficial vessels. Patients with widespread neurodermatitis (atopic dermatitis) may show these reactions more quickly, within 30-60 minutes after application, which indicates faster penetration of the cream through the skin.
In case of puncture biopsy (4 mm in diameter), application of Akriol Pro provides adequate anesthesia of intact skin in 90% of patients in 60 minutes after needle application at a depth of 2 mm and in 120 minutes after needle application at a depth of 3 mm. The efficacy of the drug is independent of skin color or pigmentation (skin type I-IV).
. When using combination vaccines against infections such as measles, rubella, mumps, or intramuscular combination vaccines against diphtheria, pertussis, tetanus, polio and infection caused by Haemophilius influenzae type b, and hepatitis B vaccination, use of the drug had no effect on the average antibody titer, the rate at which specific antibodies appeared or disappeared in serum, or the number of patients who achieved a protective or positive antibody titer after immunization.
Genital mucosal anesthesia:
Genital mucosal anesthesia is achieved faster compared to that of intact skin due to faster absorption of the drug.
In women 5-10 minutes after application of the drug to the genital mucosa anesthesia is achieved sufficient to relieve the pain caused by the use of the argon laser; the duration of anesthesia is 15-20 minutes (taking into account individual characteristics from 5 to 45 minutes).
Trophic ulcers of the lower extremities:
After application of the drug in the treatment of trophic ulcers of the lower extremities, the duration of anesthesia is up to 4 hours. No negative effects of the drug on the healing process of ulcers or with respect to bacterial flora have been noted.
Indications
Indications
In adults:
– superficial anesthesia of the skin during injections (including vaccinations), punctures and catheterization of blood vessels and superficial surgical interventions, including minor cosmetic procedures and hair removal;
– superficial anesthesia of trophic ulcers of the lower extremities during surgical treatment (mechanical cleaning), for example, to remove fibrin, pus and necrotic tissue;
– superficial anesthesia of the mucous membrane of the genital organs before painful manipulations and for pain relief before injections of local anesthetics.
In children:
– superficial anesthesia of the skin during injections (including vaccinations), punctures and catheterization of blood vessels and superficial surgical interventions (including removal of molluscum contagiosum).
Pharmacological effect
Pharmacological effect
Akriol Pro is a combination drug that contains lidocaine and prilocaine, amide-type local anesthetics. Skin anesthesia is caused by the penetration of lidocaine and prilocaine into the layers of the epidermis and dermis. The degree of anesthesia depends on the dose of the drug and the duration of application.
Intact skin:
After applying the drug to intact skin for 1-2 hours, the duration of anesthesia after removing the occlusive dressing is 2 hours. There were no differences in effectiveness (including the time to achieve an analgesic effect) and safety when using the drug on intact skin in elderly (65-96 years old) and younger patients.
Due to the effect of the drug on superficial vessels, temporary paleness or redness of the skin is possible. Similar reactions in patients with widespread neurodermatitis (atopic dermatitis) may occur faster, within 30-60 minutes after application of the drug, which indicates faster penetration of the cream through the skin.
For puncture biopsy (4 mm in diameter), the use of Acriol Pro provides adequate anesthesia of intact skin in 90% of patients 60 minutes after application of the drug when the needle is inserted to a depth of 2 mm and after 120 minutes when the needle is inserted to a depth of 3 mm. The effectiveness of the drug does not depend on the color or pigmentation of the skin (skin type I-IV).
When using combination vaccines against infections such as measles, rubella, mumps, or intramuscular combination vaccines against diphtheria, pertussis, tetanus, polio and infection caused by Haemophilius influenzae type b, as well as vaccination against hepatitis B, the use of the drug did not affect the average antibody titer, the rate of appearance or disappearance of specific antibodies in the blood serum, or the number of patients achieving a protective or positive antibody titer after immunization.
Mucous membrane of the genital organs:
Anesthesia of the genital mucosa is achieved faster compared to anesthesia of intact skin due to faster absorption of the drug.
In women, 5-10 minutes after applying the drug to the mucous membrane of the genital organs, anesthesia sufficient to relieve pain caused by the use of an argon laser is achieved; The duration of anesthesia is 15-20 minutes (taking into account individual characteristics from 5 to 45 minutes).
Trophic ulcers of the lower extremities:
After applying the drug when treating trophic ulcers of the lower extremities, the duration of pain relief is up to 4 hours. There was no negative effect of the drug on the healing process of ulcers or on bacterial flora.
Special instructions
Special instructions
Patients with glucose-6-phosphate dehydrogenase deficiency or hereditary or idiopathic methemoglobinemia are more susceptible to drug-dependent methemoglobinemia.
The effectiveness of the drug in newborns during the procedure of taking blood samples from the heel has not been established.
Caution should be exercised when applying Acriol Pro around the eyes as the drug causes eye irritation. Elimination of protective reflexes can cause irritation or damage to the cornea. If the drug gets into your eyes, immediately rinse your eyes with water or 0.9% sodium chloride solution and protect your eyes until protective reflexes are restored.
Caution must be exercised when applying the drug to the skin with atopic dermatitis; Application time should be reduced (15-30 minutes).
In children under 3 months of age, the safety and effectiveness of the drug was determined after a single dose. In these children, after application of the drug, a temporary increase in blood methemoglobin levels was often observed, lasting up to 13 hours. However, the observed increase in blood methemoglobin levels is probably not clinically significant.
Patients taking class III antiarrhythmic drugs (eg, amiodarone) should be under constant observation and ECG monitoring, because Possible effect on cardiac activity.
The drug should not be applied to a damaged eardrum or in other cases of possible penetration of the drug into the middle ear.
Acriol Pro should not be applied to open wounds.
Due to the lack of data on the absorption of the drug, it is not recommended to apply the drug to the mucous membrane of the genital organs in children.
Lidocaine and prilocaine in concentrations above 0.5-2% have bactericidal and antiviral properties. In this regard, it is recommended to take special care when using the drug before subcutaneous administration of a live vaccine (for example, BCG).
Due to the lack of data, the combined use of Acriol Pro and drugs that cause methemoglobinemia is not recommended in children aged 0 to 12 months.
Active ingredient
Active ingredient
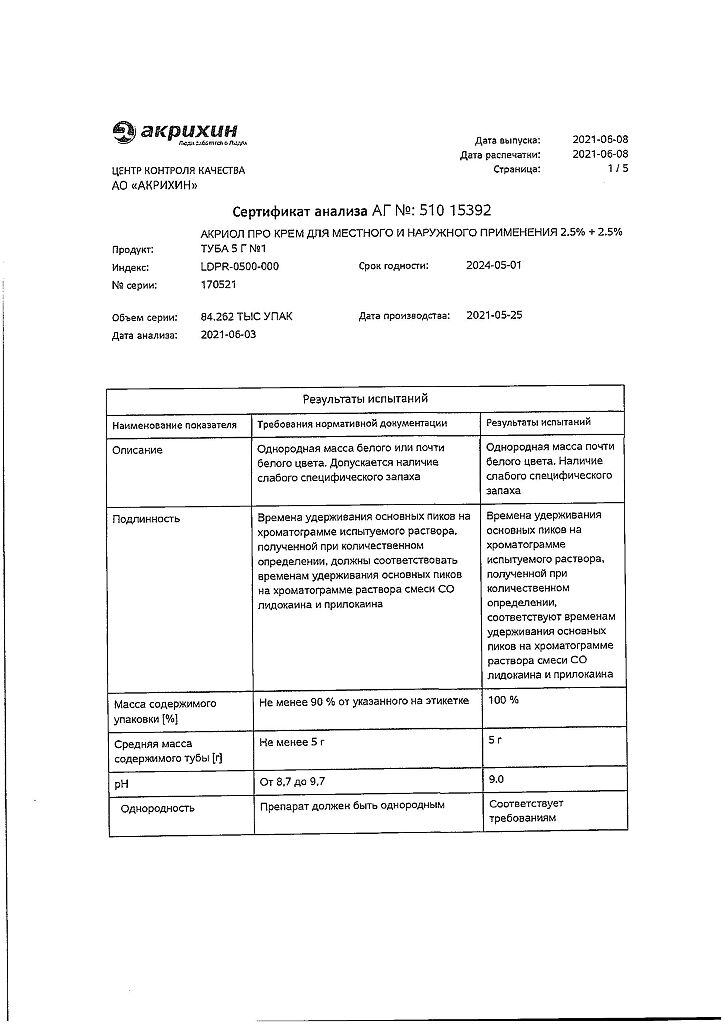
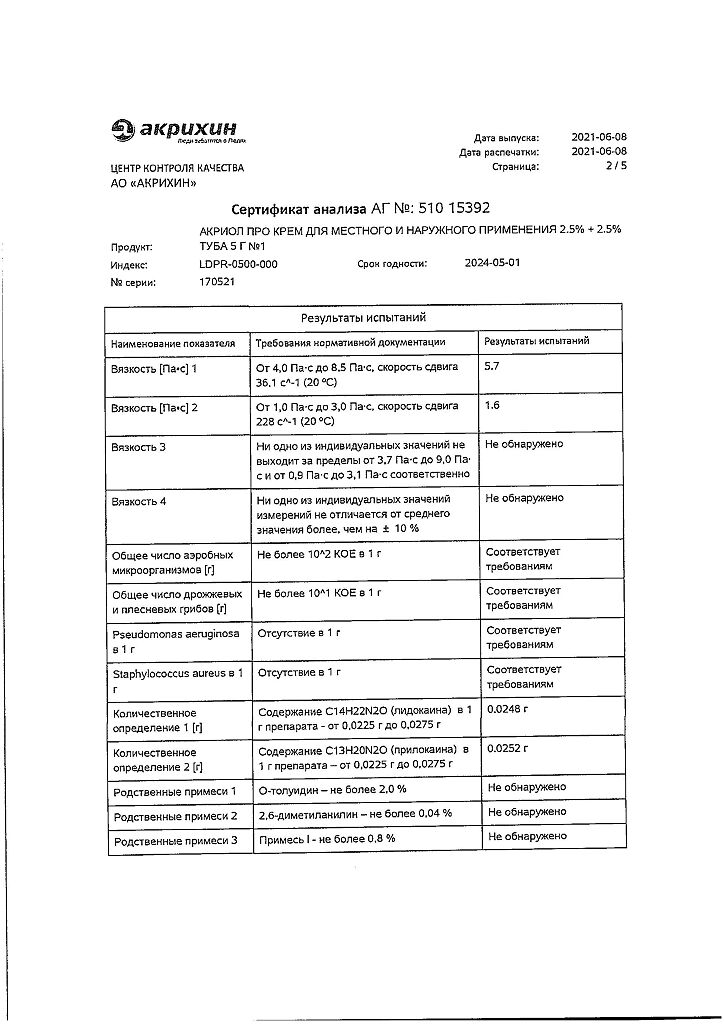
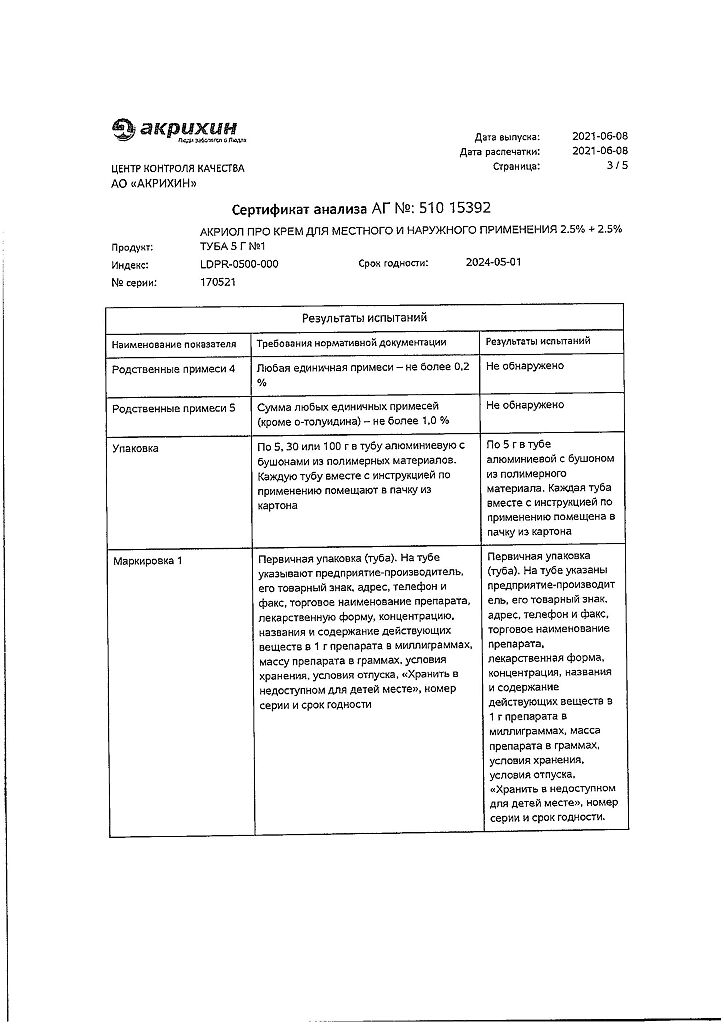
Lidocaine, Prilocaine
Composition
Composition
100 g of cream contains:
Active ingredients:
lidocaine – 2.5 g,
prilocaine – 2.5 g.
Excipients:
PEG-54 hydrogenated castor oil – 1.9 g;
carbomer – 1.0 g;
sodium hydroxide – 0.52 g;
purified water – up to 100 g.
Contraindications
Contraindications
Hypersensitivity to amide-type local anesthetics or any other component of the drug.
Premature newborns born at less than 37 weeks’ gestation. Newborns weighing less than 3 kg.
With caution: Glucose-6-phosphate dehydrogenase deficiency, hereditary or idiopathic methemoglobinemia, common neurodermatitis (atopic dermatitis), patients taking class III antiarrhythmic drugs (eg, amiodarone).
Side Effects
Side Effects
The following adverse reactions are distributed according to frequency as follows: very often (≥ 1/10); often (≥ 1/100, < 1/10); uncommon (≥ 1/1,000, < 1/100); rare (≥ 1/10,000, < 1/1,000), very rare (< 1/10,000), frequency unknown (insufficient data to estimate the frequency of development).
When applied to intact skin
Disorders of the skin and subcutaneous tissues: often – transient local reactions in the area of application of the drug, such as pallor, redness and swelling; infrequently – at the first moment after application there is a slight burning sensation, itching and a feeling of warmth (in the area where the drug was applied).
General disorders and disorders at the injection site: rarely – allergic reactions, in the most severe cases – anaphylactic shock; methemoglobinemia and/or cyanosis. Reactions in the area of application of the drug, such as hemorrhagic rash or pinpoint hemorrhages, especially after prolonged application in children with atopic dermatitis or molluscum contagiosum. Irritation of the cornea due to accidental contact of the cream with the eyes.
When applied to trophic ulcers of the lower extremities
Disorders of the skin and subcutaneous tissues: often – transient local reactions in the area of application of the drug, such as pallor, redness and swelling; at the first moment after application there is a slight burning sensation, itching and a feeling of warmth (in the area where the drug is applied); uncommon – skin irritation (in the area where the drug is applied).
General disorders and disorders at the injection site: rarely – allergic reactions, in the most severe cases – anaphylactic shock.
Interaction
Interaction
In patients receiving drugs that induce the development of methemoglobinemia (for example, drugs containing a sulfo group), Acriol Pro may increase the concentration of methemoglobin in the blood.
When treating with other local anesthetics and structurally similar drugs (including tocainide), the risk of increased systemic effects when using high doses of the drug should be taken into account.
No specific studies have been conducted to evaluate the interaction of lidocaine/prilocaine with class III antiarrhythmic drugs; caution should be exercised when using drugs together.
Pharmaceutical interaction: not detected.
Drugs that reduce the clearance of lidocaine (eg, cimetidine or beta-blockers) may cause potentially toxic plasma concentrations when repeated high doses of lidocaine are administered over an extended period of time. This interaction has no clinical significance during short-term therapy with lidocaine (including Acriol Pro) in recommended doses.
Overdose
Overdose
If the recommended dosage regimen is followed, the development of signs of systemic toxicity is unlikely.
Symptoms of intoxication are likely to be the same as with other local anesthetics, such as central nervous system (CNS) stimulation and, in severe cases, CNS and cardiac depression.
In rare cases, the development of clinically significant methemoglobinemia has been observed. Prilocaine in high doses can cause an increase in methemoglobin levels. Superficial application of prilocaine 125 mg for 5 hours caused the development of moderate methemoglobinemia in a 3-month-old child. Superficial application of lidocaine at a dose of 8.6-17.2 mg/kg caused serious intoxication in newborns.
Treatment: Severe neurological symptoms (convulsions, depression of the central nervous system) require symptomatic treatment, including the prescription of anticonvulsants and, if necessary, artificial ventilation. In case of development of methemoglobinemia, the antidote is methylthioninium chloride (methylene blue).
Due to the slow systemic absorption of the drug, patients should be monitored for several hours after starting treatment for intoxication.
Manufacturer
Manufacturer
Akrikhin JSC, Russia
Additional information
| Manufacturer | Akrihin HFC JSC, Russia |
|---|---|
| Medication form | exterior cream |
| Brand | Akrihin HFC JSC |
Other forms…
Related products
Buy Acryol Pro, cream 2.5% + 2.5% 5 g with delivery to USA, UK, Europe and over 120 other countries.