No products in the cart.
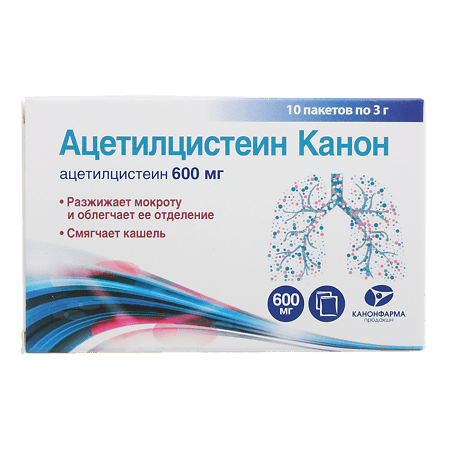
Acetylcysteine Canon, 600 mg 10 pcs
€1.00
Out of stock
(E-mail when Stock is available)
Description
Lung inflammation (pneumonia), Bronchitis, Chronic obstructive pulmonary disease, Cough, Tracheitis
Acetylcysteine is used to treat respiratory diseases accompanied by the formation of viscous sputum:
– acute and chronic bronchitis;
– obstructive bronchitis;
– tracheitis;
p> – laryngotracheitis;
– pneumonia;
– pulmonary abscess;
– bronchiolitis;
– bronchiectatic disease;
– bronchial asthma;
– cystic fibrosis
– Chronic obstructive pulmonary disease (COPD);
– acute and chronic sinusitis;
– middle ear inflammation (otitis media).
Indications
Indications
Acetylcysteine is used to treat respiratory diseases accompanied by the formation of viscous, difficult to separate sputum:
• acute and chronic bronchitis;
• obstructive bronchitis;
• tracheitis;
• laryngotracheitis;
• pneumonia;
• lung abscess;
• bronchiolitis;
• bronchiectasis;
• bronchial asthma;
• cystic fibrosis
• chronic obstructive pulmonary disease (COPD);
• acute and chronic sinusitis;
• inflammation of the middle ear (otitis media).
Pharmacological effect
Pharmacological effect
Mucolytic agent
Special instructions
Special instructions
When treating patients with diabetes, it is necessary to take into account that the drug contains sucrose (1 sachet of Acetylcysteine Canon 600 mg corresponds to 0.19 XE, 200 mg – 0.23 XE, 100 mg – 0.24 XE).
When working with the drug, you must use glass containers and avoid contact with metals, rubber, oxygen, and easily oxidized substances.
When using acetylcysteine, cases of severe allergic reactions such as Stevens-Johnson syndrome and toxic epidermal necrolysis (Lyell’s syndrome) have been very rarely reported. If changes occur in the skin and mucous membranes, you should immediately consult a doctor and stop taking the drug.
You should not take the drug immediately before bedtime (it is recommended to take the drug before 18.00).
Active ingredient
Active ingredient
Acetylcysteine
Composition
Composition
1 packet of 100 mg dosage contains:
active substance:
acetylcysteine 100 mg
excipients:
strawberry flavor 9 mg,
ascorbic acid 12.5 mg,
aspartame 15 mg,
sucrose 2863.5 mg.
1 package with a dosage of 200 mg contains:
active substance:
acetylcysteine 200 mg
excipients:
strawberry flavor 9 mg,
ascorbic acid 25 mg,
aspartame 20 mg,
sucrose 2746 mg.
1 package with a dosage of 600 mg contains:
active substance:
acetylcysteine 600 mg
excipients:
strawberry flavor 9 mg,
ascorbic acid 75 mg,
aspartame 30 mg,
sucrose 2286 mg.
Contraindications
Contraindications
– Hypersensitivity to acetylcysteine and other components of the drug;
– Sucrase/isomaltase deficiency, fructose intolerance, glucose-galactose malabsorption;
– Phenylketonuria;
– Children up to 14 years of age (for a dosage of 600 mg), children up to 6 years of age (for a dosage of 200 mg), children up to 2 years of age (for a dosage of 100 mg);
– Pregnancy and breastfeeding period.
With caution
Bronchial asthma, hepatic and/or renal failure, adrenal diseases, varicose veins of the esophagus, arterial hypertension, a tendency to pulmonary hemorrhage, hemoptysis, a history of gastric and duodenal ulcers, histamine intolerance (long-term use of the drug should be avoided, since acetylcysteine affects the metabolism of histamine and can lead to signs of intolerance, such as headache, vasomotor rhinitis, itching).
Side Effects
Side Effects
According to the World Health Organization (WHO), adverse effects are classified according to their frequency as follows: very common (≥1/10); often (≥ 1/100 and <1/10); uncommon (≥1/1000 and <1/100); rare (≥ 1/10,000 and <1/1000); very rare (<1/10000); frequency unknown (the frequency of events cannot be determined from the available data).
Allergic reactions: uncommon – skin itching, rash, exanthema, urticaria, angioedema, decreased blood pressure, tachycardia; very rarely – anaphylactic reactions up to anaphylactic shock, Stevens-Johnson syndrome, toxic epidermal necrolysis (Lyell’s syndrome).
From the nervous system: very rarely – headache.
From the cardiovascular system: very rarely – decreased blood pressure, increased heart rate (tachycardia).
From the respiratory system: rarely – shortness of breath, bronchospasm (mainly in patients with bronchial hyperreactivity in bronchial asthma).
From the gastrointestinal tract: uncommon – stomatitis, abdominal pain, nausea, vomiting, diarrhea, heartburn, dyspepsia.
From the senses: infrequently – tinnitus.
Other: very rarely – fever, isolated reports of bleeding due to hypersensitivity reactions, decreased platelet aggregation.
Interaction
Interaction
With the simultaneous use of acetylcysteine and antitussives, mucus stagnation may occur due to suppression of the cough reflex. Therefore, such combinations should be selected with caution.
Pharmaceutically incompatible with antibiotics (penicillins, cephalosporins, erythromycin, tetracycline and amphotericin B) and proteolytic enzymes.
Reduces the absorption of penicillins, cephalosporins, tetracycline (they should be taken no earlier than 2 hours after ingestion of acetylcysteine).
The simultaneous use of acetylcysteine and nitroglycerin can lead to an increase in the vasodilatory effect of the latter.
Upon contact with metals and rubber, sulfides with a characteristic odor are formed.
Overdose
Overdose
Symptoms: In case of an erroneous or intentional overdose, phenomena such as diarrhea, vomiting, stomach pain, heartburn and nausea are observed. To date, no severe or life-threatening side effects have been observed.
Treatment: Symptomatic.
Manufacturer
Manufacturer
Kanonpharma production CJSC, Russia
Additional information
| Manufacturer | Kanonfarma Production ZAO, Russia |
|---|---|
| Medication form | granules for preparation of oral solution |
| Brand | Kanonfarma Production ZAO |
Other forms…
Related products
Buy Acetylcysteine Canon, 600 mg 10 pcs with delivery to USA, UK, Europe and over 120 other countries.